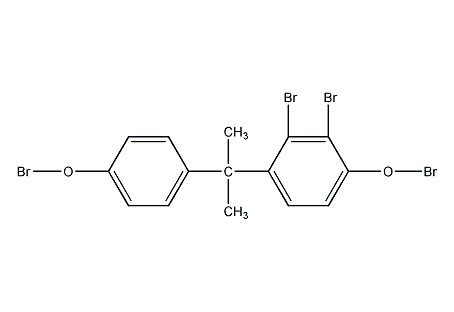
Structural formula
| Business number | 01Q9 |
|---|---|
| Molecular formula | C15H12Br4O2 |
| Molecular weight | 543.87 |
| label |
4,4′-(1-methylethylene)bis(2,6-dibromo)benzene, TBA, 4,4′-Isopropylidenebis(2,6-dibromophenol), Reactive flame retardants, Phenol, aromatic alcohols and their derivatives |
Numbering system
CAS number:79-94-7
MDL number:MFCD00013962
EINECS number:201-236-9
RTECS number:SM0894500
BRN number:1889048
PubChem number:24859851
Physical property data
1. Properties: Off-white powder.
2. Density (g/mL, 25/4℃): 2.18
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 180-184℃
5. Boiling point (ºC, normal pressure): 316℃ (decomposition)
6. Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º ): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Soluble in methanol and ether ,Insoluble in water.
Toxicological data
Rat inhalation LC: >10920 mg/m3/4H;
Mouse inhalation LC: >500 mg/m3/8H;
Rabbit skin LD50: >3160mg/ kg;
Pig inhalation LC: >500 mg/m3/8H;
2. Other multiple dose toxicity data
Rabbit skin TDL0: 100mg/kg/ 4W-I;
3. Neurotoxicity
Rabbit eye test: 100mg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 99.34
2. Molar volume (cm3/mol): 276.4
3. Isotonic specific volume (90.2K ): 728.8
4. Surface tension (dyne/cm): 48.3
5. Polarizability (10-24cm3): 39.38
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 6.8
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 21
8. Surface charge: 0
9. Complexity: 310
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Non-toxic.
Storage method
This product should be sealed and stored in a cool place. Should be stored in a clean and dry room. When transporting products, they should be protected from rain, moisture and bag breakage.
Packed in plastic bags. Store and transport according to general chemical regulations.
Synthesis method
1. First put bisphenol A and ethanol into the reaction kettle in proportion, and stir the bisphenol A into the ethanol. Then control the temperature to 25°C and add bromine while stirring. After adding the bromine, stir for 0.5h and start to pass in chlorine gas to replace the bromine in the hydrogen bromide and continue to participate in the reaction. Still maintain it at around 25°C and stir to enhance mass transfer and make the reaction proceed faster. The hydrogen chloride generated in the reaction is led to the absorber and absorbed with water to form hydrochloric acid as a by-product. After a certain amount of chlorine gas is passed through, continue stirring for half an hour, and then blow in dry air to blow out the hydrogen chloride dissolved in the reaction solution. At this time, the material was in the form of a slurry, and a large amount of tetrabromobisphenol A was supersaturated and precipitated. After cooling again, the product continues to precipitate. Filtration, the filtrate is recycled, and the filter cake is the product. After washing with water, centrifugal dehydration, and drying at about 80°C, the finished product is obtained.
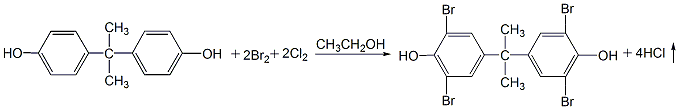
2.H2O2 can also be used as the oxidant to oxidize the HBr generated in the bromination reaction into elemental bromine, and then participate in the bromination reaction again. H2O2 can save 50% of bromine without increasing equipment investment and operation difficulty. The product quality is stable and the yield can reach 92%. The operation process is as follows: In a four-necked flask equipped with a stirrer and a thermometer, add 20g of bisphenol A, 65 mL of ethanol (95%), and 10 mL of water, and stir until the bisphenol A is completely dissolved. Keep the temperature at 23-25°C, add bromine dropwise, and when half of the bromine is added, start adding H2O2 at the same rate as the dropwise addition of bromine, and finish adding H2O2 within 0.5 hours after adding bromine. The whole process takes about 2.5 hours. Then the temperature was raised to 40°C for 2 hours, then the temperature was raised to 80°C, matured for 1 hour, and then cooled. When the material is cooled to 40°C, filter it and collect the filtrate, which will be used as a solvent next time. Wash three times with hot water at 80°C until the filtrate becomes neutral, filter with suction, and dry at 110°C to obtain the product, with a yield of 92%.
Purpose
1. Used as a flame retardant in plastics, rubber, textile, fiber and paper making industries, such as epoxy, polycarbonate, polyester, phenolic and ABS resins to make the products have good flame retardancy sex and self-extinguishing. It is currently officially used in TV high-voltage package infusion of epoxy resin.
2. This product is used as a reactive flame retardant to manufacture brominated epoxy resin, brominated phenolic resin and brominated polycarbonate. It can also be used as an additive flame retardant for polystyrene, ABS resin, AS resin and phenolic resin. This product is also used as an intermediate in the synthesis of other complex flame retardants.
3. A brominated flame retardant with a wide range of uses, which can be used as a reactive flame retardant for epoxy resins, Flame retardant of phenolic resin, polyurethane and polycarbonate; can make the product have good flame retardancy and self-extinguishing properties. It can also be used as a flame retardant for rubber, fiber, paper, etc. When used together with antimony trioxide, the flame retardant effect is better. It is also an intermediate for the synthesis of tetrabromobisphenol A ether flame retardants [such as tetrabromobisphenol A bis(hydroxyethyl) ether and octabromobisphenol ether].

 微信扫一扫打赏
微信扫一扫打赏

