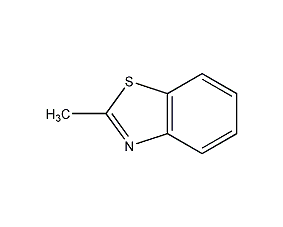
Structural formula
| Business number | 03CT |
|---|---|
| Molecular formula | C8H7NS |
| Molecular weight | 149.21 |
| label |
2-methylbenzothiazole, methylbenzothiopyridine, 2-Tolubenzothiazole, 2-Methyl-1,3-benzothiazole, 2-methylbenzothiazol, Benzothiazole,2-methyl-, USAF ek-1853, usafek-1853, 2-methylbenzothiazole, Heterocyclic compounds |
Numbering system
CAS number:120-75-2
MDL number:MFCD00005794
EINECS number:204-423-3
RTECS number:DL5600000
BRN number:112427
PubChem ID:None
Physical property data
1. Character: Light yellow crystal or liquid
2. Relative density:1.173
3. Refractive index:1.6170
4. Flashpoint (℃):102
5. Melting point (℃):14
6. Boiling point (ºC): 238
7. Boiling point (ºC,2.0kpa):150~151
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
5. Molecular property data: 1. Molar refractive index: 45.39 2. Molar volume(m3/ mol):122.5 3. isotonic ratio(90.2K):326.0 4. Surface Tension(dyne/cm)
None yet
5. Molecular property data: 1. Molar refractive index: 45.39 2. Molar volume(m3/ mol):122.5 3. isotonic ratio(90.2K):326.0 4. Surface Tension(dyne/cm):50.1 5. Dielectric constant: 6. Dipole moment(10 -24cm3): 7. Polarizability17.99 1. Reference value for hydrophobic parameter calculation (XlogP): None
Molecular structure data
Compute chemical data
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 41.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 126
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
Light yellow crystal or liquid. It smells like pyridine. Soluble in ethanol and hydrochloric acid, insoluble in water. It reacts with potassium bismuth iodide to form a red or orange-red precipitate, and reacts with antimony to form a yellow precipitate
Storage method
2. Storage:
Keep sealed in a cool place.
Synthesis method
3. Brief description of production methods
It is obtained by cyclization of thioacetanilide. Dissolve thioacetanilide in 6% sodium hydroxide solution, and add pre-cooled 20% potassium ferricyanide solution dropwise while cooling. Then, compressed air was passed through the mixed solution for 2 hours, and then left for 24 hours. The precipitated oil is extracted with diethyl ether, the extract is dried and the diethyl ether is evaporated, and the residue is distilled under reduced pressure to obtain the finished oil. The yield is 30%.
Purpose
4. Purpose
This product reacts with sodium potassium iodide to form a red or orange-red precipitate, and reacts with antimony to form a yellow precipitate. It is used as a reagent for the determination of bismuth and antimony in chemical analysis. This product is toxic and can cause allergic reactions on the skin.
i-font-family: Arial; mso-bidi-font-family: Arial”>:50.1
5. Dielectric constant:
6. Dipole moment(10 -24cm3):
7. Polarizability17.99
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 41.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 126
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
Light yellow crystal or liquid. It smells like pyridine. Soluble in ethanol and hydrochloric acid, insoluble in water. It reacts with potassium bismuth iodide to form a red or orange-red precipitate, and reacts with antimony to form a yellow precipitate
Storage method
2. Storage:
Keep sealed in a cool place.
Synthesis method
3. Brief description of production methods
It is obtained by cyclization of thioacetanilide. Dissolve thioacetanilide in 6% sodium hydroxide solution, and add pre-cooled 20% potassium ferricyanide solution dropwise while cooling. Then, compressed air was passed through the mixed solution for 2 hours, and then left for 24 hours. The precipitated oil is extracted with diethyl ether, the extract is dried and the diethyl ether is evaporated, and the residue is distilled under reduced pressure to obtain the finished oil. The yield is 30%.
Purpose
4. Purpose
This product reacts with sodium potassium iodide to form a red or orange-red precipitate, and reacts with antimony to form a yellow precipitate. It is used as a reagent for the determination of bismuth and antimony in chemical analysis. This product is toxic and can cause allergic reactions on the skin.

 微信扫一扫打赏
微信扫一扫打赏

