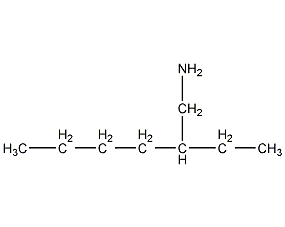
Structural formula
| Business number | 02QK |
|---|---|
| Molecular formula | C8H19N |
| Molecular weight | 129.24 |
| label |
2-Ethylhexylamine, 2-ethyl-1-hexylamine, isooctylamine, 3-(Aminomethyl)heptane, 2-Ethylhexylamine, 1-Amino-2-ethylhexane, Nitrogen-containing compound solvents, aliphatic compounds |
Numbering system
CAS number:104-75-6
MDL number:MFCD00008148
EINECS number:203-233-8
RTECS number:MQ5250000
BRN number:1209249
PubChem ID:None
Physical property data
1. Properties: colorless and transparent liquid[1]
2. Melting point (℃): -76[2]
3. Boiling point (℃): 169.2[3]
4. Relative density (water=1): 0.79[4]
5. Relative vapor density (air=1): 4.45[5]
6. Saturated vapor pressure (kPa): 0.16 (20℃) [6]
7. Octanol/water partition coefficient: 2.82[7]
8. Flash point (℃ ): 52 (OC) [8]
9. Ignition temperature (℃): 295[9]
10. Explosion upper limit (%): No data yet
11. Explosion lower limit (%): 1.1[10]
12. Solubility: soluble Soluble in water, ethanol and acetone. [11]
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 500mgREACTION SEVERITY, moderate reaction; Standard Dresser test: rabbit skin contact, 750μg/24HREACTION SEVERITY, strong reaction; Standard Dresser test: rabbit Eye contact, 50μg/24HREACTION SEVERITY, strong reaction;
2. Acute toxicity: rat oral LD50: 450mg/kg; rat inhalation LCLo: 125ppm/4H; rabbit skin contact LD50: 600μL/kg ;
3. Can irritate skin and mucous membranes.
4. Acute toxicity [12] LD50: 450mg/kg (rat oral); 600mg/kg (rabbit dermal)
p>
5. Irritation [13]
Rabbit transdermal: 750μg (24h), severe irritation.
Rabbit eye: 50μg (24h), severe irritation.
Ecological data
1. Ecotoxicity[14] IC50: 0.02~0.36mg/L (72h) (algae)
2. Biodegradability No data available
3. Non-biodegradability No data available
4. Other harmful effects[15] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 42.60
2. Molar volume (cm3/mol): 164.6
3. Isotonic specific volume (90.2K ): 376.8
4. Surface tension (dyne/cm): 27.4
5. Dielectric constant: 2.27
6. Dipole moment (10-24cm3):
p>
7. Polarizability: 16.89
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 52.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has the chemical properties of primary amines and has optical isomers. It can dissolve paraffin wax and carnauba wax when heated and solidify when cooled.
2. Stability[16] Stable
3. Incompatible substances[17] Strong oxidizing agent, strong acid
4. Polymerization hazard[18] No polymerization
5. Decomposition products[19] Ammonia
Storage method
Storage Precautions[20] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep container tightly sealed. They should be stored separately from oxidants and acids, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the reaction of 2-ethylhexanol and ammonia. In the same set of batch kettle equipment, 2-ethylhexylamine, di(2-ethylhexyl)amine and tris(2-ethylhexyl)amine can be produced in rotation.
Refining method: Add metal sodium to dehydrate and then distill.
Purpose
1. Used as raw materials for medicines, dyes, pesticides, vulcanization accelerators, antioxidants, flotation agents, emulsifiers, etc. Mainly used in the production of (2-ethylhexylamine)-1-isopropyl-4-methyl-bicyclo(2,2,2)octyl chloride-2,3-dihydroxyimide. As a pesticide synergist, it has the same synergistic effect as piperonyl butoxide.
2. Used in synthetic detergents, rubber chemicals, oil additives and pesticides, etc. [21]

 微信扫一扫打赏
微信扫一扫打赏

