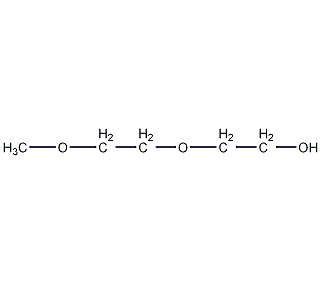
Structural formula
| Business number | 0349 |
|---|---|
| Molecular formula | C5H12O3 |
| Molecular weight | 120.15 |
| label |
Diethylene glycol methyl ether, Diethylene glycol monomethyl ether, Monomethyldiethylene glycol ether, Diethylene glycol monomethyl ether, Methyl carbitol, Diethylene glycol ether, Diethylene glycol monomethyl ether, Methyl diglycol, Methyl carbitol, CH3OC2H4OC2H4OH, hydrocarbon fuel modifiers, textile auxiliaries, hydrocarbon extraction agent, Dyes for coloring |
Numbering system
CAS number:111-77-3
MDL number:MFCD00002871
EINECS number:203-906-6
RTECS number:KL6125000
BRN number:1697812
PubChem number:24880998
Physical property data
1. Properties: Colorless liquid with slightly aromatic odor and slightly bitter taste.
2. Density (g/mL, 20/4ºC): 1.0270
3. Density (g/mL, 25/4ºC): 1.0167
4 .Relative vapor density (g/mL, air=1): 4.14
5. Melting point (ºC): -76
6. Freezing point (ºC, supercooling): < – 76
7. Boiling point (ºC, normal pressure): 193
8. Refractive index (20ºC): 1.4264
9. Refractive index (25ºC): 1.4246
10. Refractive index (40ºC): 1.4188
11. Viscosity (mPa·s, 25ºC): 3.48
12. Flash point (ºC, opening): 93
13. Ignition point (ºC): 221
14. Heat of evaporation (KJ/mol, b.p.): 46.60
15. Heat of combustion (KJ/mol): 3011.6
16. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.25
17. Vapor pressure (kPa, 25ºC): 0.024
18. Volume expansion coefficient (K-1, 20ºC): 0.00086
19. Explosion upper limit (%, V/V): 22.7
20. Lower explosion limit (%, V/V): 1.38
21. Solubility: can be mixed with water, alcohol, ether, acetone, carbon tetrachloride, glycerin, dimethyl Miscible with formamide, etc. Dissolves grease, natural resins, dyes, cellulose acetate, cellulose nitrate, polyvinyl acetate, vinyl chloride-vinyl acetate copolymer, etc.
22. Critical temperature (ºC): 398.85
23. Critical pressure (MPa): 3.67
24. Liquid phase standard hot melt (J·mol -1·K-1): 280.2
Toxicological data
1. Acute toxicity: rat oral LD50: 9210 mg/kg; rabbit dermal LD50: 650mg/kg
2. Irritation data: Eye – rabbit 500 mg/24 hours Mild.
3. It is of low toxicity and the maximum allowable concentration in the workplace is 122.75mg/m3. Animal deaths are mostly caused by deep anesthesia and kidney damage. Eye contact can sometimes cause pain.�Temporary damage. Skin irritation is not obvious, but toxic doses can be absorbed through the skin.
Ecological data
No harm to water bodies.
Molecular structure data
1. Molar refractive index: 30.23
2. Molar volume (cm3/mol): 121.3
3. Isotonic specific volume (90.2K ): 287.1
4. Surface tension (dyne/cm): 31.4
5. Polarizability (10-24cm3): 11.98
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 38.7
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 38.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
miscible with water, alcohol, ether, acetone, carbon tetrachloride, glycerol, dimethylformamide, etc. Hygroscopic. It has the chemical properties of alcohol and ether.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. This product is a by-product of the industrial production of ethylene glycol monomethyl ether. Mix ethylene oxide and methanol, add them to the reactor after preheating. The reaction temperature is 150 to 200°C. The reaction pressure is 1.96~3.92MPa, and the excess of methanol is about 15%. In addition to the ethylene glycol monomethyl ether generated by the main reaction, the reaction product also contains diethylene glycol methyl ether and triethylene glycol methyl ether generated by the side reaction, and excess methanol. The four main components are separated through the alcohol recovery tower and three vacuum distillation towers. The methanol obtained is recycled and products such as ethylene glycol monomethyl ether, diethylene glycol methyl ether and triethylene glycol methyl ether are obtained.

Refined method : Dry with anhydrous sodium sulfate or calcium chloride and then fractionate.
2.Mainly adopt the ethylene oxide method and the ethylene glycol production by-product recovery method. Ethylene oxide method: Hydrolyze ethylene oxide to form diethylene glycol ether, which is then reacted with methanol. Ethylene glycol production by-product recovery method: react the scraps from ethylene glycol production with methanol and obtain it by distillation under reduced pressure.
Purpose
1. This product is used as a high-boiling point solvent for resins, cellulose, coatings, inks, and dyes, and as a diluent for adhesives. Added to paint to make it easier to flow, paint and level. Can be used as an extraction agent for hydrocarbons. It is used as an intermediate for the preparation of ester derivatives in the organic synthesis industry, brake fluid, and as a chemical reagent in analytical chemistry. Used as a solvent for nitrocellulose, resin, wood coloring dyes, stamp inks, printing inks, alcohol-soluble dyes, etc. Others are also used as hydrocarbon fuel modifiers and intermediates in the preparation of ester derivatives.
2. Cosmetic solvents. It is mainly used as the main solvent for cosmetics such as nail polish and is an excellent solvent for cellulose.

 微信扫一扫打赏
微信扫一扫打赏

