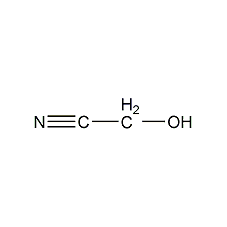
Structural formula
| Business number | 02UU |
|---|---|
| Molecular formula | C2H3NO |
| Molecular weight | 57.05 |
| label |
Ethanol cyanide, Hydroxyacetonitrile, Hydroxyacetonitrile, Glycolonitrile, Formaldehyde Cyanohydrin, hydroxyethanol, cyanomethanol, Hydroxyacetonitrile, 2-Hydroxyacetonitrile, 2-Hydroxymethylnitrile, cyanomethanol, Hydroxyacetonitrile, Glycolonitrile, Glycolic acid nitrile, Formaldehyde cyanhydrin, Formaldehyde cyanohydrin |
Numbering system
CAS number:107-16-4
MDL number:MFCD00042731
EINECS number:203-469-1
RTECS number:AM0350000
BRN number:605328
PubChem number:24873384
Physical property data
1. Properties: colorless oily liquid.
2. Density (g/mL, 20℃): 1.076
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -67
5. Boiling point (ºC, normal pressure): 183
6. Boiling point (ºC, 3.2kPa): 119
7. Refractive index (D20): 1.389
8. Flash point (ºC): Undetermined
9. Specific rotation (ºC): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, 20ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: soluble in ethanol and ether.
Toxicological data
1. Acute toxicity: Rat oral LD50: 8mg/kg; rat inhalation LCLo: 27ppm/8H; mouse oral LD50: 10mg/kg; mouse inhalation LCLo: 27ppm/8H; mouse Transabdominal LD50: 3mg/kg; Mouse subcutaneous LDLo: 15mg/kg; Rabbit skin contact LD50: 5mg/kg; Rabbit eye LDLo:13mg/kg;
Ecological data
None
Molecular structure data
1. Molar refractive index: 12.76
2. Molar volume (cm3/mol): 52.4
3. Isotonic specific volume (90.2K ): 136.7
4. Surface tension (dyne/cm): 46.2
5. Polarizability (10-24cm3): 5.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.7
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 44
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 41.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
This product is highly toxic. It is easily absorbed through the skin and is easily decomposed into formaldehyde and hydrocyanic acid, so it is almost as toxic as hydrocyanic acid and can cause headaches, dizziness, nausea, vomiting, weakness, ataxia, and elevated body temperature. Cyanide can be found in the blood. The oral LD50 for rats is about 8 mg/kg. It is very toxic to the human body. Even if it is not inhaled, it can be fatal when it is splashed on clothes. The workplace should have good ventilation, equipment should be sealed, and operators should wear protective equipment.
Storage method
This product is stored and transported according to the regulations on toxic and dangerous goods.
Synthesis method
1. Obtained from the reaction of formaldehyde and potassium cyanide. Slowly add 37% formaldehyde aqueous solution dropwise to the potassium cyanide aqueous solution, and control the temperature not to exceed 10°C. After standing for 10 minutes, while still stirring below 10°C, add dilute sulfuric acid until the pH is 1.9. Then add 5% potassium hydroxide solution dropwise until the pH is about 3.0, then add diethyl ether, and filter out the generated potassium sulfate. The filtrate was extracted with diethyl ether. After drying, the ether is evaporated, the residue is distilled under reduced pressure, and the fraction at about 100°C (1.33kPa) is collected to obtain the finished product. 2. Produced by the reaction of formaldehyde and hydrocyanic acid in the presence of a catalyst. In addition, hydroxyacetonitrile can also be produced from ethanol and ammonia through gas phase catalysis.

Purpose
Organic synthetic raw materials. It can be used as an intermediate for the production of glycine, malononitrile and indigo dye.

 微信扫一扫打赏
微信扫一扫打赏

