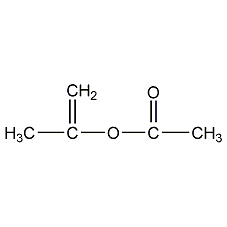
Structural formula
| Business number | 02WN |
|---|---|
| Molecular formula | C5H8O2 |
| Molecular weight | 100.12 |
| label |
1-methylvinyl acetate, Isopropenyl acetate, Isopropylene acetate, Acetic Acid Isopropenyl Ester |
Numbering system
CAS number:108-22-5
MDL number:MFCD00008709
EINECS number:203-562-7
RTECS number:UD4200000
BRN number:1280347
PubChem number:24846886
Physical property data
1. Properties: colorless transparent liquid
2. Density (g/mL, 25℃): 0.91
3. Relative vapor density (g/mL, air = 1): 3.45
4. Melting point (ºC): -92.9
5. Boiling point (ºC, normal pressure): 92-94
6. Boiling point (ºC, kPa): Not determined
7. Refractive index (D20): Not determined
8. Flash Point (ºC): 18
9. Specific rotation (ºC): Undetermined
10. Autoignition point or ignition temperature (ºC): 431
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturation vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/ Log value of the distribution coefficient (water): Undetermined
17. Explosion upper limit (%, V/V): 7.8
18. Explosion lower limit (%, V/V): 1.8
19. Solubility: miscible in alcohol, ether, ketone, etc.
Toxicological data
1. Skin/eye irritation
Standard Draize test: rabbit, skin contact: 500mg/24H, severity of reaction: mild.
Standard Draize test: Rabbit, eye contact: 500 mg, severity of reaction: moderate.
Standard Draize test: Rabbit, eye contact: 500mg/24H, severity of reaction: mild.
2. Acute toxicity: Rat oral LD50: 3mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 26.55
2. Molar volume (cm3/mol): 109.4
3. Isotonic specific volume (90.2K ): 240.9
4. Surface tension (dyne/cm): 23.5
5. Polarizability (10-24cm3): 10.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: None
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 94.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain chemical bond stereocenters Number of centers: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, alkalis, and acids.
2. Highly volatile and flammable chemicals are recommended to be stored in a dry and low-temperature place and used in a fume hood.
Storage method
1. Usually products contain polymerization inhibitors. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
The ketene gas obtained by cracking acetic acid or acetone undergoes an addition reaction with the acetylsulfonyl acetic acid-acetone solution. The addition liquid is subjected to rough distillation and fractionation to obtain isopropylene acetate.
Purpose
1. Used as raw materials for polymers and organic synthesis.
2. Isopropylene acetate is mainly used in the acetyl transfer reaction with hydroxyl compounds in organic synthesis to generate the corresponding acetate; and to convert the carbonyl group into enol acetate, resulting The product can also undergo further cyclization reactions and condensation reactions.
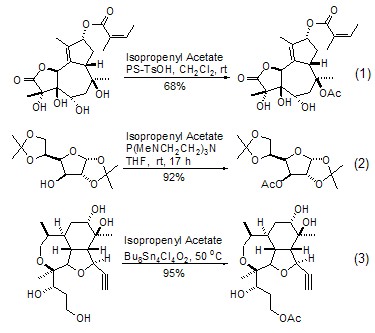
The most commonly used reaction of isopropylene acetate is the acetyl transfer reaction with hydroxyl compounds under various catalytic conditions to generate the corresponding acetate ester. Acidic catalytic conditions are the simplest, but the yield and selectivity are not very ideal (Formula 1)[1]. If an organophosphine compound catalyst is used, the reaction can be carried out under neutral conditions and gives an almost quantitative yield (formula 2)[2]. Organotin catalysts not only give excellent yields under neutral conditions but also achieve high regioselectivity in the presence of multiple hydroxyl groups. The main factor affecting selectivity is steric hindrance, and the priority of selectivity is primary alcohol > secondary alcohol > tertiary alcohol (formula 3)[3,4]. Using other catalysts, structural factors can also be used to bring selectivity to achieve selective esterification of alcoholic hydroxyl groups in the presence of phenolic hydroxyl groups[5].
Isopropylene acetate in biology Acetyl transfer reactions with hydroxyl compounds under enzymatic conditions have become an important application of this reagent. By selecting appropriate biological enzymes and catalytic conditions, the result of a single enantiomer can be obtained (Formula 4)[6,7]. Sometimes the reaction speed of this reaction is very different from that of the enantiomers, so it can be used for the chiral kinetic resolution of alcohol compounds (Formula 5)[8]. An interesting result is that under enzyme-catalyzed conditions, when isopropylene acetate undergoes an acetyl transfer reaction, multiple chiral centers in the molecule are all transformed (Formula 6)[9].
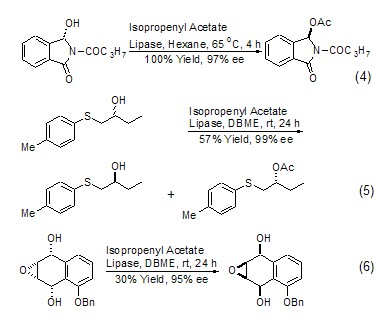
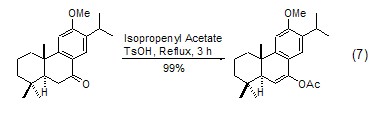
Isopropylene Acetate Another A less important reaction is the conversion of the carbonyl group to enol acetate. If both sides of the carbonyl group have the possibility of forming an enol structure, isomers are often produced. Generally, this reaction is carried out under the action of an acidic catalyst, and a variety of acidic catalysts can be used for this purpose, such as: H2SO4, TsOH, etc. (Formula 7 )[10,11].

 微信扫一扫打赏
微信扫一扫打赏

