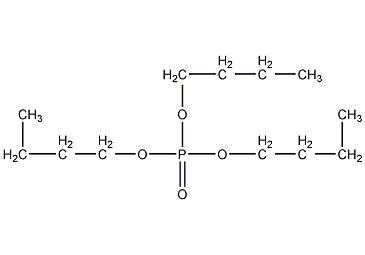
Structural formula
| Business number | 03K8 |
|---|---|
| Molecular formula | C12H27O4P |
| Molecular weight | 266.31 |
| label |
n-butyl phosphate, Tri-n-butyl phosphate, Tri-n-butyl phosphate, Tributyl phosphate, Tri-n-butyl phosphate, 3-Butyl phosphate, Phosphate 3-butyric acid, TBP, defoamer, paint stripper, uranium extraction agent, heat exchange medium, plasticizer, Electronic coating raw materials and intermediates |
Numbering system
CAS number:126-73-8
MDL number:MFCD00009436
EINECS number:204-800-2
RTECS number:TC7700000
BRN number:1710584
PubChem number:24889413
Physical property data
1. Properties: Colorless, almost odorless liquid.
2. Boiling point (ºC, 1.33kPa): 289
3. Melting point (ºC): -80
4. Relative density (g/mL, 20/4ºC): 0.9766
5. Relative vapor density (g/mL, air=1): 8.86
6. Refractive index (25ºC): 1.4224
7. Flash point (ºC, open): 146
8. Heat of vaporization (J/g): 230.7
9. Ignition point (ºC): 204
10. Viscosity (mPa·s): 3.7
11. Heat of evaporation (KJ/mol): 72.0
12. Heat of generation (KJ/mol): 1455.8
13. Heat of combustion (KJ/mol): 7979.6
14. Solubility: Slightly soluble in water, 165mL of water can dissolve 1mL of tributyl phosphate. Miscible with a variety of organic solvents.
15. Freezing point (ºC): -80
Toxicological data
1. Acute toxicity: Oral – rat LD50: 3000 mg/kg; Oral – mouse LC50: 1189 mg/kg
2. Irritation data: Skin – rabbit 10 mg/24 hours; Eyes – Rabbit 500 mg Severe
3. It has a strong irritating effect on the skin and respiratory tract, and has systemic toxic effects. Large doses administered orally or intraperitoneally can cause collapse, pulmonary edema, and convulsions. Excitatory effect on the central nervous system. It has a mild inhibitory effect on cholinesterase in human blood and plasma. The maximum allowable concentration in the workplace is 5 mg/m3. The oral LD50 in rats is about 8.0mL.
生Ethological data
It has a strong irritating effect on the skin and respiratory tract, and has systemic toxic effects. It has a mild inhibitory effect on cholinesterase in human blood and plasma. There is a danger of burning when exposed to high heat, open flame or contact with oxidants. It decomposes when heated to produce highly toxic phosphorus oxide fumes.
Molecular structure data
1. Molar refractive index: 69.75
2. Molar volume (cm3/mol): 269.8
3. Isotonic specific volume (90.2K ): 640.6
4. Surface tension (dyne/cm): 31.7
5. Polarizability (10-24cm3): 27.65
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: none
6. Topological molecule polar surface area 44.8
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 175
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product is a flammable, colorless, odorless liquid that decomposes when heated to produce highly toxic phosphorus oxide fumes.
Chemical properties: Adding dry hydrogen chloride at room temperature produces chlorobutane. In the presence of boron trifluoride, it reacts with benzene to produce sec-butylbenzene and 1,4-di-sec-butylbenzene. Treatment with aniline and dilute sodium hydroxide produces dibutyl aniline.
Storage method
Store in a cool and ventilated place. Store and transport according to regulations on toxic chemicals.
Synthesis method
1. Prepared by the reaction of phosphorus oxychloride (POCl3) and n-butanol.
Add butanol into the esterification pot, cool it to below 10°C, add phosphorus oxychloride while stirring, keep the reaction humidity at about 30°C, and stir until the reaction is completed. Add water to wash, let stand to separate the water layer, and then neutralize with 10% sodium carbonate solution until the pH value is 7. Control the humidity below 40°C. The neutralized crude ester is distilled under reduced pressure and dealcoholized, and then washed with water to reduce the acidity. When the requirements are met, then distill under reduced pressure, remove low boiling matter and collect the 150-180°C/1.333-0.667kPa fraction to obtain the product.
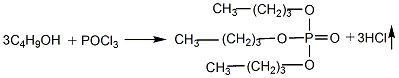
2.Slowly add phosphorus oxychloride into n-butanol, stir with pressurized air, the temperature should not exceed 40°C, stir for 3 hours after addition, and discharge hydrogen chloride.
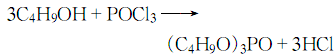
After letting it cool, add it to cold salt water, suck off the bottom layer, and wash it again with cold salt water. Add the washed acidic dibutyl ester to water, neutralize it with saturated sodium carbonate solution to a pH value of 6 to 7, then add saturated salt water to separate the salt solution. Then add anhydrous sodium sulfate and shake for 3 hours, let it stand overnight, and carry out fractionation under reduced pressure to obtain the finished product.
3. Preparation method:
In a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and dropping funnel, add anhydrous n-butanol ( 2) 222g (3.0mol), 260g pyridine (3.3mol), anhydrous benzene 300mL. Cool to -5°C in an ice-salt bath while stirring, and slowly add 153g (1.0mol) of phosphorus oxychloride dropwise. Control the reaction temperature not to exceed 10°C. After the addition is completed, slowly heat to reflux and maintain the reaction at this temperature for 2 hours. After cooling, add 500 mL of water and use the solvent pyrrole salt ①. Separate the benzene layer and wash with water. Dry over anhydrous sodium sulfate. Distill under reduced pressure and collect the fraction at 160~162℃/2,0kPa to obtain 190~200g of tri-n-butyl phosphate ② (1), with a yield of 71% to 75%. Note: ① Pyridine hydrochloride is soluble in water. The water layer is concentrated under reduced pressure, then neutralized with sodium hydroxide, and the pyridine layer is distilled. About 50% of the pyridine can be recovered. ② Referring to the above method and replacing butanol with n-propanol, the following various trialkyl phosphates can be obtained (Table I-9-4). [1]
Purpose
1. Used as the main plasticizer of nitrocellulose, cellulose acetate, chlorinated rubber and polyvinyl chloride. It is also commonly used as a solvent, defoaming agent, antistatic agent, and extractant for rare earth elements in coatings, adhesives and inks. Used as a solvent for nitrocellulose, cellulose acetate, and ink. It is also used as defoaming agent, paint remover, uranium extraction agent, heat exchange medium, plasticizer, etc.
2.Gas chromatography stationary solution. Used to extract cobalt, iridium, manganese, molybdenum, palladium, platinum, rhodium, technetium, uranium and tungsten. Colorimetric determination of molybdenum. Used as a solvent, it is also often used as a plasticizer for nitrocellulose, cellulose acetate, chlorinated rubber and polyvinyl chloride.
3.Used as analytical reagents, such as extraction agents, solvents, and gas chromatography stationary solutions. It is also used as plastic plasticizer and organic synthesis intermediate.
4.A defoaming agent for various adhesives, with strong foam breaking ability, but poor foam suppression performance. It can also be used as a defoaming agent for emulsion paints, inks, cement, etc.; as a metal extractant in hydrometallurgy of uranium, rare earth elements, zirconium, etc.; as an additive for cellulose acetate, nitrocellulose, polyvinyl chloride, chlorinated rubber, etc. Plastic agents; rubber flame retardants; anti-scorch agents, etc. It is also used as a solvent for coatings, inks, etc., an additive for lubricating oils, and a heat transfer medium. It is also used in the manufacture of herbicides, insecticides and fungicides.
Metal extractants during hydrometallurgy of acetic acid, zirconium, etc.; plasticizers for cellulose acetate, nitrocellulose, polyvinyl chloride and chlorinated rubber; rubber flame retardants; anti-scorch agents, etc. It is also used as a solvent for coatings, inks, etc., an additive for lubricating oils, and a heat transfer medium. It is also used in the manufacture of herbicides, insecticides and fungicides.

 微信扫一扫打赏
微信扫一扫打赏

