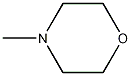
Structural formula
| Business number | 02YH |
|---|---|
| Molecular formula | C5H11NO |
| Molecular weight | 101.15 |
| label |
N-methylmorpholine, N-methylmorpholine, 4-methylmorpholine, 4-methylmorpholine, 1,4-Oxaazepane, N-Methyl morphofine, N-Methylmorpholine, 4-Methyl-1-oxa-4-azacyclohexane, Heterocyclic compounds |
Numbering system
CAS number:109-02-4
MDL number:MFCD00006175
EINECS number:203-640-0
RTECS number:QE5775000
BRN number:102719
PubChem number:24865480
Physical property data
1. Properties: colorless liquid with ammonia odor. [1]
2. Melting point (℃): -66[2]
3. Boiling point (℃): 115~116[3]
4. Relative density (water=1): 0.92[4]
5. Relative vapor density (air=1): 3.5[5]
6. Saturated vapor pressure (kPa): 2.39 (20℃)[6]
7. Octanol/water partition coefficient: -0.33[7]
8. Flash point (℃): 24 (OC)[8]
9. Solubility: miscible with water, soluble in benzene. [9]
Toxicological data
1. Skin/eye irritation
Open irritation test: rabbit, skin contact: 400mg, severity of reaction: mild.
Standard Draize test: Rabbit, Eye contact: 920 μg, Severity of reaction: Severe.
Standard Draize test: Rabbit, eye contact: 20mg/24H, severity of reaction: mild.
2. Acute toxicity:
Oral LD50 in rats: 1960mg/kg; LDLo in rats by inhalation: 2000ppm/4H;
Oral in mice LC50: 1970mg/kg; mouse inhalation LC50: 25200mg/m3/2H;
Rabbit skin contact LD50: 1350μL/kg; mammal oral LD50: 1917mg /kg;
3. Acute toxicity[10]
LD50: 1960mg/kg (rat oral); 1242mg/kg (rabbit transdermal)
LC50: 25200mg/m3 (mouse inhalation, 2h)
4. Irritation[11]
Rabbit transdermal: 460mg, mild irritation (open irritation test) .
Rabbit eye: 920μg, severe irritation.
Ecological data
1. This substance is slightly harmful to water.
2. Ecotoxicity[12] TLm: 159~499mg/L (24~96h) (fish)
3. Biodegradability No information available
4. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 28.32
2. Molar volume (cm3/mol): 108.5
3. Isotonic specific volume (90.2K): 247.2
4. Surface tension (dyne/cm): 26.9
5. Polarizability: 11.22
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 12.5
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 50
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Administration to animals by any route will cause short-term sexual excitement, and then turn to inhibition and atrophy. Inhaling the vapor of this product can still stimulate mucous membranes and cause spasms in animals. The tissue changes caused by poisoning with this product include malnutrition of the liver and kidneys. After inhalation by mice for 2 hours, its LC50: 25.5mg/L. Rat gavage LD50: 1.97mg/kg. According to the changes in the total capacity of the central nervous system, the threshold concentration for one action is 0.09~0.1mg/L. The threshold concentration for stimulating effect on humans is 0.2mg/L. The irritating effect on skin is 1/1.5 of morpholine. The maximum allowable concentration in the air is 5mg/m3. This product is toxic and irritating to human skin and mucous membranes. The production workshop should have good ventilation and the equipment should be sealed. Operators should wear protective equipment. See morpholine (D059).
2. Stability[13] Stable
3. Incompatible substances[14] Acids, acid anhydrides, strong oxidants, carbon dioxide
4. Conditions to avoid contact [15] Heat
p>
5. Hazards of aggregation[16] No aggregation
Storage method
Storage Precautions[17] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of morpholine with formaldehyde and formic acid. Slowly add formaldehyde to morpholine, add formic acid dropwise while stirring, the reaction will automatically reflux and release carbon dioxide. After adding formic acid, heat to reflux for 4-5 hours. After cooling, add sodium hydroxide and distill immediately. Collect all fractions less than 99°C. Add sodium hydroxide until saturated. Cool and separate the oil layer. Dry and fractionate. Collect the fractions between 114.5-117°C to become the finished product. Another method uses dichloroethyl ether, monomethylamine aqueous solution, liquid caustic soda and other raw materials to synthesize in one step. The process is short, the equipment is simple, the process conditions are mild, the number of investments is small, the product yield is high and the quality is good.
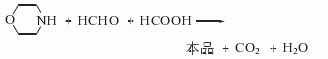
Purpose
1. Used in the production of extraction solvents, stabilizers for chlorinated hydrocarbons, corrosion inhibitors, catalysts, and drugs.
2. Used as chromatographic reagents, solvents, stabilizers, catalysts, and extractants. Used in organic synthesis and pharmaceutical industry.
3. Used as solvent, catalyst, corrosion inhibitor, and also used in the synthesis of rubber vulcanization accelerator and other fine chemicals. Also used as a catalyst for the synthesis of ampicillin and carbenicillin.
4. Used as catalysts, extractants, stabilizers for chlorinated hydrocarbons, corrosion inhibitors, analytical reagents and in pharmaceutical manufacturing. [18]

 微信扫一扫打赏
微信扫一扫打赏

