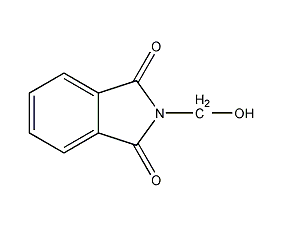
Structural formula
| Business number | 039M |
|---|---|
| Molecular formula | C9H7NO3 |
| Molecular weight | 177.16 |
| label |
Phthalimidomethanol, pesticides |
Numbering system
CAS number:118-29-6
MDL number:MFCD00005899
EINECS number:204-241-4
RTECS number:None
BRN number:140946
PubChem number:TI5270000
Physical property data
1. Properties: White crystalline powder.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 147-149
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, KPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Hardly soluble in general organic solvents
Toxicological data
1. Reproductive toxicity: TDLO in female rats after fertilization: 300mg/kg, lasting for 13 days
Ecological data
Slightly harmful to water.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 43.87
2. Molar volume (cm3/mol): 121.1
3. Isotonic specific volume (90.2K): 346.2
4. Surface tension (dyne/cm): 66.6
5. Polarizability (10-24cm3): 17.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 57.6
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 227
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of stereocenters of chemical bonds���:0
15. Number of covalent bond units: 1
Properties and stability
1. Stable at room temperature and pressure, avoid contact with strong oxidants.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and water sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Obtained from the reaction of phthalimide and formaldehyde. Add formaldehyde to the reaction pot and add water to prepare the formaldehyde to a concentration of about 12%. Add phthalimide under stirring, the dosage is phthalimide:formaldehyde=1:1.1 (molar ratio). Raise the temperature to 98-102°C, reflux for 2.5 hours, cool to 35°C, centrifuge, filter and dry to obtain the finished product.
2.1. Preparation method using water as solvent [1].
Prepared from 100 grams of phthalic acid Grind phthalimide into fine powder, suspend it in 80 ml of 40% formaldehyde, add 200 ml of water, heat to 103-108°C and reflux for 4 hours. The reaction is complete when all of the phthalimine has dissolved. If necessary, filter while it is hot and let the filtrate cool. Once N is methylphthalimide, it will precipitate into colorless glittering flake crystals.
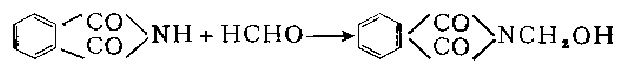
3.. Preparation method using ethanol as solvent
3 grams (0.0204 mol) of phthalimide are suspended in 35 ml of boiling 80% ethanol. Add 2 ml of 37% formaldehyde solution and reflux until phthalylimine is completely dissolved. Most of the ethanol is evaporated, that is, the product crystals are precipitated and recrystallized in water or benzene.
Purpose
1. Used in the organic synthesis of pesticides, dyes, pigments, and medicines.
2.Preparation of N-derivatives of phthalimide, such as reaction with primary aromatic amines to produce N-(aromatic aminomethyl) phthalimide Phthalimide.

 微信扫一扫打赏
微信扫一扫打赏

