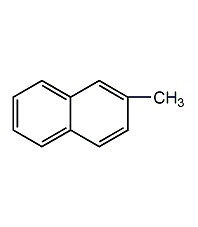
Structural formula
| Business number | 023P |
|---|---|
| Molecular formula | C11H10 |
| Molecular weight | 142.20 |
| label |
β-methylnaphthalene, β-Methylnaphthalene, aromatic compounds |
Numbering system
CAS number:91-57-6
MDL number:MFCD00004118
EINECS number:202-078-3
RTECS number:QJ9635000
BRN number:906859
PubChem number:24869412
Physical property data
1. Properties: white to light yellow monoclinic crystal or molten solid. [1]
2. Melting point (℃): 34~36[2]
3. Boiling point (℃) :241.1[3]
4. Relative density (water = 1): 1.03[4]
5. Critical Pressure (MPa): 3.5[5]
6. Octanol/water partition coefficient: 3.86[6]
7. Flash point (℃): 97 (CC) [7]
8. Ignition temperature (℃): 529[8]
9. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, and benzene. [9]
10. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5864.4
11. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): 106.7
12. Gas phase standard entropy (J·mol-1 ·K-1): 380.03
13. Gas phase standard free energy of formation (kJ·mol-1): 211.3
14. Gas phase standard hot melt (J·mol-1·K-1): 159.8
15. Liquid phase standard claims heat (enthalpy )(kJ·mol-1): 56.78
16. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -5802.71
17. Crystal phase standard claim heat (enthalpy) (kJ·mol-1): 44.94
18. Crystal phase standard entropy (J ·mol-1·K-1): 1219.99
19. Crystal phase standard formation free energy (kJ·mol-1): 192.82
20. Crystal phase standard hot melt (J·mol-1·K-1): 196.0
Toxicological data
1. Acute toxicity[10] LD50: 1630mg/kg (rat oral)
2. Irritation[11] Rabbit transdermal: 0.05 ml (24h), severe stimulation.
Ecological data
1. Ecotoxicity[12] LC50: 9mg/L (96h) (fish)
2. Biodegradability[13] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, 72% degradation after 28 days.
3. Non-biodegradability[14] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 7.4h (theoretical).
4. Bioconcentration[15] BCF: 407 (blue gill Sunfish, exposure concentration 0.013μg/L, exposure time 26d)
Molecular structure data
1. Moore’s foldRatio: 48.92
2. Molar volume (cm3/mol): 139.8
3. Isotonic specific volume (90.2K): 348.7
4. Surface tension (dyne/cm): 38.7
5. Dielectric constant (F/m): 2.78
6. Polarizability (10-24cm3): 14.83
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 128
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances [17] Strong oxidizing agent
3. Conditions to avoid contact[18] Heating
4. Polymerization hazard[19] No polymerization
Storage method
Storage Precautions[20] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 35℃. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
2-Methylnaphthalene mainly exists in the naphthalene oil fraction (content about 5.48%) and wash oil fraction (content about 8.11%). 1. Distill the methylnaphthalene fraction into a narrow fraction that can be crystallized at 237-241°C, and use the freeze crystallization method to prepare 2-methylnaphthalene. Melt it at 40-45°C and filter it to obtain 2-methylnaphthalene with a purity of more than 97%. 2. Contains about 1.0%-1.8% in high-temperature tar. Use the washing oil in coal tar as raw material, remove phenol, remove pyridine alkali and then rectify to cut out the 240-245°C methylnaphthalene fraction, freeze it to -20°C, at this time, 2-methylnaphthalene will crystallize and precipitate. Then use diaphoresis crystallization method or methanol or ethanol recrystallization for refinement to obtain the product.
Purpose
1. Used in medicine as an intermediate for the production of vitamin K3, and oxidized to prepare β-naphthol for long-acting or short-acting oral contraceptives. In agriculture, it is used as a synthetic plant growth regulator and DDT emulsifier; after sulfonation, it can be used as a detergent; it can also be used as a raw material for fiber dyeing aids, wetting agents, surfactants, pesticides, etc.
2. Used in organic synthesis, and also used in the production of pesticides, vitamin K, dyes, etc. [21]

 微信扫一扫打赏
微信扫一扫打赏

