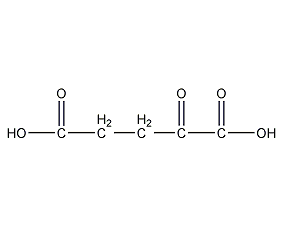
Structural formula
| Business number | 0485 |
|---|---|
| Molecular formula | C5H6O5 |
| Molecular weight | 146.1 |
| label |
a-oxoglutaric acid, alpha-ketoglutaric acid, 2-oxo-1,5 glutaric acid, α-glutinic acid, 2-Oxo-1,5-pentanedioic acid, a-Ketoglutaric acid, acidic solvent |
Numbering system
CAS number:328-50-7
MDL number:MFCD00004165
EINECS number:206-330-3
RTECS number:None
BRN number:1705689
PubChem ID:None
Physical property data
1. Physical property data
1. Properties: white fine crystalline powder. It becomes light grayish yellow after long storage and is easy to deliquesce.
2. Density (g/mL, 25/4℃): Not available
3. Relative vapor density (g/mL, Air=1): Not available
4. Melting point (ºC): 113.5~115
5. Boiling point (ºC, normal pressure) : 345.6
6. Boiling point (ºC, 5.2kPa): Not available
7. Refractive index: Not available
8. Flash point (ºC): 177
9. Specific rotation (º): Not available
10. Autoignition point or ignition temperature (ºC): Not available
11. Vapor pressure (kPa, 25ºC): Not available
12. Saturated vapor pressure (kPa, 60ºC): Not available
13. Heat of combustion (KJ/mol): Not available
14. Critical temperature (ºC): Not available
15. Critical pressure (KPa): Not available
16. Oil and water (octanol /water) logarithmic value of the distribution coefficient: not available
17. Explosion upper limit (%, V/V): not available
18 .Lower explosion limit (%, V/V): Not available
19. Solubility: Easily soluble in water, alcohol, extremely difficult to dissolve in ether.
Toxicological data
2. Toxicological data:
Acute toxicity: Not available.
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 28.37
2. Molar volume (cm3/mol): 97.4
3. Isotonic specific volume (90.2K ): 279.7
4. Surface tension (dyne/cm): 67.9
5. Polarizability (10-24cm3):11.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.9
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 5
4.���Number of rotational chemical bonds: 4
5. Number of tautomers: 2
6. Topological molecule polar surface area 91.7
7. Heavy atoms Number: 10
8. Surface charge: 0
9. Complexity: 171
10. Number of isotope atoms: 0
11. Determine the number of stereocenters of atoms: 0
12. Determine the number of stereocenters of atoms: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Exist in tobacco leaves and smoke.
Storage method
None yet
Synthesis method
1. Mix 225g triethyl oxalosuccinate and 600ml concentrated hydrochloric acid, and let the mixture pass. Distill and concentrate to 140°C, and the residue is cooled and crystallized to obtain 110-112g of α-ketoglutaric acid, with a yield of 92-93%.
2. Preparation method:
Triethyl oxalylsuccinate (3): In a reaction bottle equipped with a stirrer and reflux condenser, add 360 mL of absolute ethanol , add 23g (1.0mol) of clean metallic sodium in batches, and heat and reflux after the addition to complete the reaction of the metallic sodium. Change to a distillation device, evaporate the ethanol, and continuously add toluene dropwise at the same time to remove the ethanol until the internal temperature reaches 105°C. Cool to room temperature, add 650 mL of anhydrous ether and 146 g (1 mol) of diethyl oxalate, and add while stirring Diethyl succinate (2) 174g (1.0mol), left at room temperature for 12 hours. Add 500 mL of water while stirring, separate the organic layer, extract the organic layer with 150 mL of water, combine the aqueous layers, and acidify with concentrated hydrochloric acid to precipitate an oily substance. The oil layer was separated, and the water layer was extracted three times with diethyl ether. The organic layers were combined and dried over anhydrous magnesium sulfate. The diethyl ether was recovered to obtain 235-250g of yellow oily compound (3), with a yield of 86%-91%. a-Carbonylglutaric acid (1): In a reaction bottle equipped with a stirrer and a reflux condenser, add 225g (0.82mol) of the above compound (3), 330mL of concentrated hydrochloric acid, and 660mL of water, and heat to reflux for 4 hours while stirring. Concentrate to dryness (60-70°C), add 200 mL of nitroethane to the residue, heat to dissolve, filter while hot, and wash the filter cake with hot nitroethane. Combine the nitroethane solutions and stir at 0 to 10°C for 5 hours. After suction filtration and drying under reduced pressure at 90°C for 4 hours, 88 to 99 g of compound (1) was obtained, with a yield of 73% to 83%.
Purpose
Substrate for determination of aminoconvertase and dehydrogenase, supporting reagent for determination of liver function, and also used as an intermediate in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

