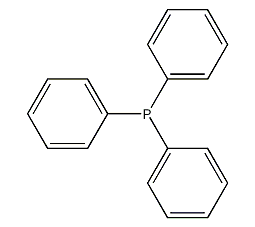
Structural formula
| Business number | 067S |
|---|---|
| Molecular formula | C18H15O3P |
| Molecular weight | 262 |
| label |
None yet |
Numbering system
CAS number:603-35-0
MDL number:MFCD00003043
EINECS number:210-036-0
RTECS number:SZ3500000
BRN number:610776
PubChem ID:None
Physical property data
1. Character: white crystal[15]
2. Melting point (℃): 80.5[16]
3. Boiling point (ºC): 377[17]
4. Relative density (water = 1): 1.194 (25ºC) [18]
5. Relative vapor density (air=1): 9.0[19]
6. Critical pressure (MPa): 7.84[20 ]
7. Octanol/water partition coefficient: 5.69[21]
8. Flash point (ºC): 180 (OC )[22]
9. Solubility: insoluble in water, slightly soluble in ethanol, soluble in benzene, acetone, carbon tetrachloride, easily soluble in ether. [23]
Toxicological data
1. Acute toxicity[24]
LD50: 700mg/kg (rat oral)
LC50 : 12167mg/m3 (rat inhalation, 4h)
2. Irritation [25]
Rabbit transdermal: 500mg (24h), mild stimulation.
Rabbit eye: 500mg (24h), mild irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: not available
2. Molar volume (cm3/mol): not available
3. etc. Zhang specific volume (90.2K): None available
4. Surface tension (dyne/cm): None available
5. Dielectric constant: None available
6. Polarizability (10-24cm3): None available
7. Single isotope mass: 262.091136 Da
8. Nominal mass: 262 Da
9. Average mass: 262.2855 Da
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 202
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It will irritate the human body under severe exposure to the sun. It will be neurotoxic if exposed for a long time. It is a dangerous item and cannot coexist with strong oxidizing agents. Arylphosphines are less reactive with oxygen than benzyl and alkylphosphines. However, the oxidation of triphenylphosphine by air is very obvious, resulting intriphenylphosphine oxide. Triphenylphosphine is not prone to fire and explosion, but when it is heated and decomposes, it will generate toxic phosphine and POxsmoke. Operation should be carried out in a fume hood.
2. Stability[26] Stable
3. Incompatible substances[27] Strong oxidizing agent
4. Conditions to avoid contact[28] Heat
5.Polymerization hazard[29] No polymerization
6. Decomposition products[30] Phosphorus Alkane
Storage method
Storage Precautions[31] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Under a dry atmosphere, prepare the Grignard reagent using 34.1 g (0.22 mol) of olfactory benzene, 5.2 g (0.21 g atom) of magnesium and 108 ml of anhydrous ether. The resulting phenyl bromide Store the magnesium under an inert gas, stir and cool with an ice bath, slowly add (15 to 20 minutes) 6.0 g (0.044 mol) phosphorus trichloride and 35 ml of diethyl ether solution. Use 11 ml of concentrated hydrochloric acid and 30 ml of water. The mixture was slowly treated with the solution. The ether layer was separated, and the water layer was extracted with ether. After the ether solutions were combined, distill under normal pressure hydrogen until the temperature of the residue in the distillation vessel reached 285°C. The residue was recrystallized with ethanol. times, 8.7 grams (76%) of white triphenyl was obtained, with a melting point of 79.5°C.
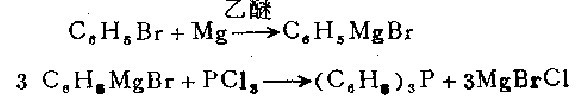
Purpose
1. Used in organic synthesis, polymerization initiator, raw material of antibiotic drug clindamycin, and standard sample for organic trace analysis to determine phosphorus.
2. Preparation of palladium, iridium, rhodium, nickel and other complex catalysts, Wfttig reagent, triphenylphosphine dihalide deoxygenation (pyridine N-oxide, nitrosobenzene, hydroperoxide), Desulfurization and debromination reagents. A bromo nitro compound generates nitrile. React with fatty diazo compounds to synthesize a_ketoaldehyde and β-ketoester. Beckmann rearrangement. Dequaternization of pyridine bell salts. It is used in some synthesis together with bromine iodine, carbon tetrachloride (bromide), N-butadiene imine bromide, etc.
3. Triphenylphosphine is a quite commonly used reducing agent. In most cases, the reaction is driven by the formation of triphenylphosphine oxide (a thermodynamically favorable reaction). In addition, triphenylphosphine is widely used as a ligand for metal catalysts.
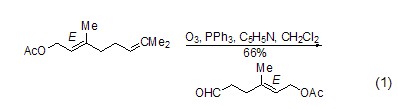
Deoxygenation reaction Triphenylphosphine is widely used in the reduction of hydrogen peroxide or endoperoxide. The reaction is dependent on the substrate and can generate alcohols, carbonyl compounds or cyclic compounds. Oxide. The main driving force for this type of reaction is the ability of triphenylphosphine to form strong P=O bonds with relatively weak O-O bonds (188~209 kJ/mol). For example, triphenylphosphine can be used to reductively decompose ozonides and selectively prepare ketones and aldehydes (formula 1)[1].
With azide Reaction Triphenylphosphine reacts with organic azide compounds to form iminophosphine (formula 2)[2].
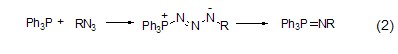
Iminophosphonane is a A more active nucleophile that easily reacts with electrophiles. For example, it reacts with aldehydes and ketones to generate imines and triphenylphosphine oxides. This reaction is similar to the Wittig reaction and is called the aza-Wittig reaction. The driving force of this reaction is also due to the generation of triphenylphosphine oxide (formula 3)[3].
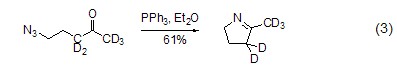
With organic sulfides Reaction Triphenylphosphine can convert episulfide compounds into alkenes (formula 4)[4] at room temperature.

Dehalogenation reaction α-Bromoketone reacts with triphenylphosphine to form ketone (Formula 5)[5].
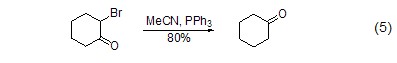
With organic epoxy Reaction of compoundsRefluxing in water and acetone solvents, triphenylphosphine can convert epoxy compounds into cyclic imines with the participation of sodium azide (Formula 6)[6] .
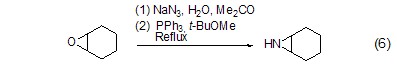
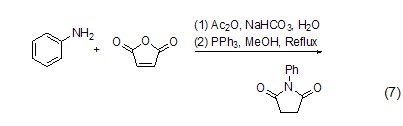
Preparation of substituted pyrrole Aniline, furandione and triphenylphosphine react to form 1-phenyl-2,5-pyrroledione (formula 7)[7].
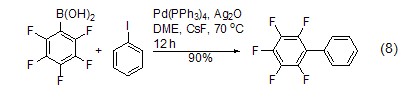
As a metal catalyst Ligand It serves as a ligand to form a metal catalyst with many transition metals, such as Pd(PPh3)4, which is an important catalyst[8 ], often used in catalytic coupling reactions, is an important method to construct carbon-carbon bonds, and is characterized by mild catalytic conditions. For example, in Pd(PPh3)4 and Ag2O, phenylboronic acid reacts directly with aromatic halogenated hydrocarbons to form biphenyl compounds. The yield of this reaction reaches 90% (Equation 8 )[9,10]. In addition to phenylboronic acid and halogenated compounds, magnesium reagents [11], zinc reagents [12], tin reagents [13], silicon compounds [14], etc. can be used as substrates for coupling reactions.
4. Widely used in medicine, In petrochemical, coating, rubber and other industries, it is used as catalyst, accelerator, flame retardant, and also used as analytical reagent. [32]

 微信扫一扫打赏
微信扫一扫打赏

