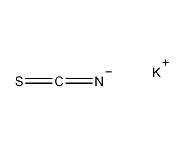
Structural formula
| Business number | 049G |
|---|---|
| Molecular formula | KSCN |
| Molecular weight | 97.18 |
| label |
potassium thiocyanate, Potassium sulfocya-nate, Potassium rhodanide, refrigerant, indicator, stripping agent, Inorganic corrosion inhibitor materials |
Numbering system
CAS number:333-20-0
MDL number:MFCD00011413
EINECS number:206-370-1
RTECS number:XL1925000
BRN number:3594799
PubChem ID:None
Physical property data
1. Properties: colorless monoclinic crystal.
2. Density (g/mL, 25/4℃): 1.886
3. Melting point (ºC): 172.3
4. Boiling point (ºC, Normal pressure): 500
5. Flash point (ºC): 500
6. Solubility: soluble in water, ethanol and acetone.
7. Refractive index: 1.660
Toxicological data
Acute toxicity: LD50: 590 mg/kg (orally in mice); 850 mg/kg (orally in rats)
When large doses cause acute poisoning, it can cause nausea, vomiting, and abdominal pain. , diarrhea and other gastrointestinal dysfunction, blood pressure fluctuations, and slow heart rate.
Maximum allowable concentration in air: 50mg/m3
Ecological data
This substance is harmful to the environment. Special attention should be paid to the pollution of water bodies.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 24.8
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 34.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. Semi-hydrated crystals (KSCN·0.5H2O) can be obtained at low temperatures, which are stable at -29.5~6.8℃, turn blue when heated to about 430℃, but turn colorless again after cooling. Decomposes when heated to 500°C. When in contact with ferrous salts, blood-red ferric thiocyanide is produced, but in the presence of ferrous salts, there is no reaction. poisonous. Solubility g/100g water 177 (0℃), 239 (25℃), 673.6 (99℃).
Storage method
1. Should be stored in a cool, dry place. Pay attention to moisture-proof, do not press, and prevent opening the lid and breaking the barrel.
2. Keep away from fire and heat sources. Do not store or transport together with acids and foods.
Combinedmethod
1. It can be obtained by the reaction of potassium cyanide and sulfur or by the pressure synthesis reaction of carbon disulfide and ammonia water to generate ammonium thiocyanate, which is then reacted with potassium carbonate. The ammonium thiocyanate conversion method carries out a pressurized synthesis reaction between carbon disulfide and ammonia water to generate ammonium thiocyanate and by-product ammonium hydrogen sulfide, and then through desulfurization and evaporation, the ammonium hydrogen sulfide is decomposed into hydrogen sulfide and removed. It starts slowly at the liquid temperature of 105°C. Add potassium carbonate solution to generate potassium thiocyanate. A large amount of carbon dioxide and ammonia will be produced during the reaction. Ammonia can be recycled and used. The reaction solution is filtered to remove insoluble matter, evaporated under reduced pressure, and then cooled, crystallized, and centrifuged to obtain industrial potassium thiocyanate. The reaction formula is as follows: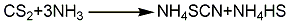
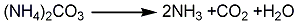 2. The crude product is obtained by the metathesis reaction of potassium salt and ammonium thiocyanate. The purification method is to recrystallize multiple times in boiling ethanol, and then at 100 ℃, dry under reduced pressure and store in a desiccator filled with concentrated sulfuric acid. 3.Use ammonium thiocyanate conversion method. Sulfur dioxide and ammonia are synthesized under pressure to generate ammonium thiocyanate and by-product ammonium thiocyanate. The ammonium thiocyanate is decomposed into hydrogen sulfide and removed through desulfurization and evaporation. Potassium carbonate solution is slowly added when the liquid temperature is 105°C, that is Potassium thiocyanate is generated.
2. The crude product is obtained by the metathesis reaction of potassium salt and ammonium thiocyanate. The purification method is to recrystallize multiple times in boiling ethanol, and then at 100 ℃, dry under reduced pressure and store in a desiccator filled with concentrated sulfuric acid. 3.Use ammonium thiocyanate conversion method. Sulfur dioxide and ammonia are synthesized under pressure to generate ammonium thiocyanate and by-product ammonium thiocyanate. The ammonium thiocyanate is decomposed into hydrogen sulfide and removed through desulfurization and evaporation. Potassium carbonate solution is slowly added when the liquid temperature is 105°C, that is Potassium thiocyanate is generated.
Purpose
1. Analytical reagent, used to identify ferric iron, copper, silver and conduct urine tests. It is also an indicator for legal titanium. It is used as stripping agent and refrigerant in the electroplating industry; it can also be used in the dye industry, photography industry, pesticides and other fields.
2.Packaged in plastic barrels lined with polyethylene plastic bags, with net weight of 25kg or 40kg per barrel. This product is easily deliquescent and should be stored in a ventilated, dry and clean warehouse. Do not pressure. Protect from rain and sunlight during transportation. Do not store or transport together with acid or food.
3.Used for cyanide copper plating, cyanide silver plating and removal of nickel plating on copper and copper-tin alloy substrates It is mainly used as an additive in solutions such as black nickel plating and silver plating electrolyte.
4. Used as corrosion inhibitor in detergents.

 微信扫一扫打赏
微信扫一扫打赏

