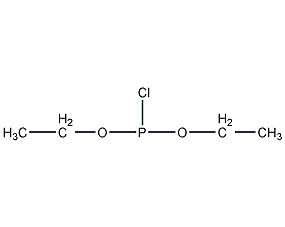
Structural formula
| Business number | 0608 |
|---|---|
| Molecular formula | C4H10ClO2P |
| Molecular weight | 156.55 |
| label |
Diethyl phosphorochloridite, Diethyl chlorophosphonite, (C2H5O)2PCl |
Numbering system
CAS number:589-57-1
MDL number:MFCD00009074
EINECS number:209-652-2
RTECS number:None
BRN number:1098392
PubChem number:24894269
Physical property data
1. Physical property data
1. Boiling point (ºC, normal pressure): 153-155 °C
2. Density: 1.082g/cm3
3. Solubility: Soluble in benzene, tetrahydrofuran, ether, dichloromethane and acetonitrile.
Toxicological data
None yet
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 18.5
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 47.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability and reactivity:
Materials to avoid: reactive metals, moisture, moisture, alkali, oxides.
Products to be decomposed: carbon monoxide and carbon dioxide, phosphorus oxides, hydrogen chloride.
2. Flammable liquid, highly toxic, sensitive to moisture, needs to be stored in nitrogen. Generally performed in a fume hood.
Storage method
Storage:
Seal the container and store it in a sealed main container in a cool, dry place.
Synthesis method
It is prepared by the direct reaction of phosphorus trichloride and triethyl phosphite under the action of absolute ethanol or sodium ethoxide.
Purpose
None

 微信扫一扫打赏
微信扫一扫打赏

