
Structural formula
| Business number | 01PP |
|---|---|
| Molecular formula | C4H8O2 |
| Molecular weight | 88.11 |
| label |
2-Methylpropionic acid, 2-Methylpropionic acid, Iaobutanoic acid, 2-Methylpropionic acid, acid solvents, aliphatic compounds |
Numbering system
CAS number:79-31-2
MDL number:MFCD00002658
EINECS number:201-195-7
RTECS number:NQ4375000
BRN number:635770
PubChem number:24895944
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): -47[2]
3. Boiling point (℃): 154.5[3]
4. Relative density (water = 1): 0.95[4]
5. Relative vapor Density (air=1): 3.04[5]
6. Saturated vapor pressure (kPa): 0.2 (20℃)[6]
7. Heat of combustion (kJ/mol): -2165.3[7]
8. Critical temperature (℃): 336[8]
9. Critical pressure (MPa): 4.05[9]
10. Octanol/water partition coefficient: 0.5~1.13[10]
11. Flash point (℃): 55.6 (OC); 77 (CC) [11]
12. Ignition temperature (℃): 481[12]
13. Explosion limit (%): 10[13]
14. Lower explosion limit (%): 2[14]
15. Solubility: insoluble in water, miscible in ethanol, ether, chloroform, glycerin, Propylene glycol, etc. [15]
16. Refractive index (n20ºC): 1.393
17. Refractive index (n25ºC): 1.393020
18. Viscosity (mPa·s, 25ºC): 1.213
19. Viscosity (mPa·s, 30ºC): 1.126
20. Heat of evaporation ( KJ/mol, 25ºC): 57.11
21. Heat of evaporation (KJ/mol, b.p.): 44.46
22. Heat of fusion (KJ/mol): 5.02
23. Vapor pressure (kPa, 25ºC): 0.19
24. Critical density (g·cm-3): 0.304
25. Critical volume (cm3·mol-1): 290
26. Critical compression factor: 0.213
27. Eccentricity Factor: 0.618
28. Solubility parameter (J·cm-3)0.5: 23.157
29. van der Waals area (cm2·mol-1): 7.870×109
30.van der Waals volume ( cm3·mol-1): 53.860
31. Liquid phase standard hot melt (J·mol-1 ·K-1): 174.6
Toxicological data
1. Acute toxicity
Rat oral LD50: 280 uL/kg; rabbit skin LD50: 500 uL/kg;
2. Neurotoxicity
Rabbit skin test: 149ug/24HREACTION;
3. It is of low toxicity. Same as propionic acid. Moderately irritating to skin and eyes, no allergic effects. The oral LD50 in rats is 400~800mg/kg.
4. Acute toxicity [16]
LD50: 280μl (266mg)/kg (rat oral); 500μl (475mg)/kg (rabbit transdermal)
5. Stimulate� Sex[] Rabbit transdermal: 139μg (24h), causing irritation (open stimulation test)
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[17] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: 22.10
2. Molar volume (cm3/mol): 89.5
3. Isotonic specific volume (90.2K ): 210.4
4. Surface tension (dyne/cm): 30.4
5. Polarizability (10-24cm3): 8.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 56.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[18] Stable
2. Incompatible substances[19] Alkali, strong oxidizing agent, strong reducing agent
3. Polymerization hazard[20] No polymerization
Storage method
Storage Precautions[21] Stored in a cool, ventilated warehouse. The storage temperature should not exceed 37℃. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Direct oxidation method of isobutyraldehyde: isobutyraldehyde can be obtained by direct oxidation reaction with air or oxygen.
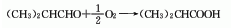
2. Methacrylic acid hydrogenation The reaction formula of the method is as follows:
![]()
3 .Preparation method:
Into a 5L reaction bottle equipped with a stirrer, dropping funnel, and thermometer, add 52g (0.7mol) isobutanol (2), 150mL of 10% sodium carbonate aqueous solution, and ice-water bath Cool, control the temperature at 10 to 15°C, and add dropwise a solution of 142g (0.9mol) of potassium permanganate dissolved in 2.75L of water, and finish the addition in about 3 to 4 hours. After adding, leave it at room temperature overnight①. Filter off the precipitated manganese dioxide, and concentrate the filtrate under reduced pressure to about 150 mL. After cooling, use 50% sulfuric acid to acidify to pH 2-3. Extract with ether (50 mL × 3), dry over anhydrous magnesium sulfate, evaporate the ether, and collect the fraction between 150 and 158°C by distillation. This fraction was redistilled once, and the fraction between 153 and 155°C was collected to obtain 45 g of isobutyric acid (1)②, with a yield of 76%. Note: ① The purple color of potassium permanganate should be faded. If it does not fade, you can continue stirring for a longer time, or add a small amount of methanol to make the purple color disappear. ② It can also be prepared by oxidizing isobutanol with potassium dichromate. Dimethylmalonic acid is heated to 190°C and loses one molecule of carbon dioxide to generate isobutyric acid. [23]
Purpose
1. Isobutyric acid is not as important as butyric acid. The uses are similar to those of n-butyric acid, and are mainly used to produce corresponding esters. For example, methyl isobutyrate has an apricot aroma, propyl isobutyrate has a pineapple aroma, isoamyl isobutyrate has a banana aroma, and octyl isobutyrate has a grape aroma. Benzyl isobutyrate has a strawberry-like jasmine flavor, and can be used as synthetic flavors and solvents. Also used in the manufacture of varnishes and plasticizers. Isobutyric acid has some important derivatives, which are actually used in industry as intermediates in the production of isobutyronitrile, and then converted into isobutyramidine hydrochloride, which is the raw material for the pesticide diazinon.
2. Used to synthesize isobutyrate products, such as methyl isobutyrate, propyl ester, isopentyl ester, benzyl ester, etc., which can be used as edible spices and pharmaceuticals. [22]

 微信扫一扫打赏
微信扫一扫打赏

