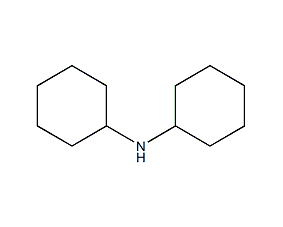
Structural formula
| Business number | 02L6 |
|---|---|
| Molecular formula | C12H23N |
| Molecular weight | 181.32 |
| label |
N-cyclohexylcyclohexylamine, dicyclohexylamine, Dodecahydrodiphenylamine, Dicyclohexylamine, Bis(Cyclohexyl)Amine, N,N-Diclohexylamine, Ester cyclic compounds and their derivatives |
Numbering system
CAS number:101-83-7
MDL number:MFCD00011658
EINECS number:202-980-7
RTECS number:HY4025000
BRN number:605923
PubChem number:24851183
Physical property data
1. Properties: Colorless and transparent liquid with fishy odor. [1]
2. Melting point (℃): -0.1[2]
3. Boiling point (℃): 256[3]
4. Relative density (water = 1): 0.91[4]
5. Relative vapor Density (air=1): 6.27[5]
6. Saturated vapor pressure (kPa): 1.60 (37.7℃)[6]
7. Critical pressure (MPa): 2.52[7]
8. Octanol/water partition coefficient: 4.370[8]
9. Flash point (℃): 99 (OC) [9]
10. Ignition temperature (℃): <230[10]
11. Explosion upper limit (%): 5.6[11]
12. Explosion lower limit (%): 0.6[12]
13. Solubility: Slightly soluble in water, miscible in ethanol, ether and benzene. [13]
Toxicological data
1. Skin/eye irritation:
Standard Dresser test: rabbit skin contact, 2mg/24 HREACTION SEVERITY, strong reaction;
Standard Dresser test: rabbit Eye contact, 750μg/24HREACTION SEVERITY, strong reaction;
2. Acute toxicity:
Rat oral LD50: 373mg/kg;
Mouse Oral LD50: 500mg/kg;
Mouse subcutaneous LD50: 135mg/kg;
Rabbit subcutaneous LDLo: 500mg/kg;
3. Chronic toxicity/ Carcinogenicity:
Oral TDLo in rats: 40mg/kg/52W-I;
Subcutaneous TDLo in mice: 2404mg/kg/48W-I;
4. Mutagenicity: cell generation analysis test: human leukocytes, 200μg/L;
5. Can be absorbed by the skin and is more toxic than cyclohexylamine. Its vapor is a spasmodic poison and can cause death when inhaled in large quantities.
6. Acute toxicity [14] LD50: 373mg/kg (rat oral)
7 . Irritation[15]
Rabbit transdermal: 2mg (24h), severe irritation.
Rabbit eye: 750μg (24h), severe irritation.
8. Mutagenicity[16] Cytogenetic analysis: human leukocytes 200 μg/L
9 .Carcinogenicity [17] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals.
Ecological data
1. Ecotoxicity[18] LC100: 20mg/L (48h.�� (High body yarrow)
2. Biodegradability [19] Activated sludge method, initial concentration 100mg/L, 2 After the week, it was downgraded by 76.9%.
3. Non-biodegradability [20] The half-life of photolysis is 2.9d (theoretical)
4. Bioconcentration[21] BCF: 1200 (theoretical)
Molecular structure data
1. Molar refractive index: 57.12
2. Molar volume (cm3/mol): 198.2
3. Isotonic specific volume (90.2K ): 475.8
4. Surface tension (dyne/cm): 33.1
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 22.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 12
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 116
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: It has the chemical properties of secondary amines. It is highly alkaline and can form salts with various acids. React with acid chloride, acid anhydride, ester, etc. to obtain N-acylate. React with p-methanesulfonyl chloride to obtain sulfonamide compounds. Reacts with nitrous acid to form nitrosamines. Halogen-substituted derivatives can be obtained by reacting with oxychloride, sulfonyl chloride, phosphorus trichloride, silicon tetrachloride, etc. In addition, dicyclohexylamine easily forms hydrates and alcoholates containing crystal water with water and ethanol respectively.
2. This product is highly toxic. It has a strong penetrating odor and is therefore easier to detect. Mouse LD502g/kg, rat LD503.49g/kg. Dicyclohexylamine can be absorbed through the skin, causing skin allergies and gangrene. The vapor can cause nausea and anesthesia, but it will not cause blood poisoning. Dicyclohexylamine has been reported to cause cancer. The maximum allowable concentration in the air in the workplace is 10mg/m3. Production equipment should be sealed to prevent running, emitting, dripping and leaking. There is forced ventilation at the operation site, and operators wear protective equipment.
3. Stability[22] Stable
4. Incompatible substances[23] Acids, acid chlorides, non-oxidizing acids, strong oxidants, chloroform
5. Polymerization hazards[24] No aggregation
Storage method
1. Storage precautions [25] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Sealed packaging in iron drums, net weight 150kg. Store in a cool, ventilated and dry place, isolated from fire sources. Loading, unloading and transportation should be carried out in accordance with the regulations for flammable and toxic chemicals.
Synthesis method
Using aniline as raw material and hydrogenating it under high temperature and high pressure in the presence of a catalyst, dicyclohexylamine is produced.
Refining method: fractional distillation and refining.

Purpose
1. Used in organic synthesis, and also used as pesticides, acid gas absorbers and steel rust inhibitors. Used as extraction agent for natural products and organic synthesis, and acid gas absorbent. The salt formed with fatty acids and sulfuric acid has excellent surface activity and is used in the printing and fiber industries. The complex formed with metal is used as a catalyst for coatings and inks. Dicyclohexylamine nitrite is an excellent metal corrosion inhibitor.
2. Used in organic synthesis and as pesticides, acid gas absorbers, and steel rust inhibitors. [26]

 微信扫一扫打赏
微信扫一扫打赏

