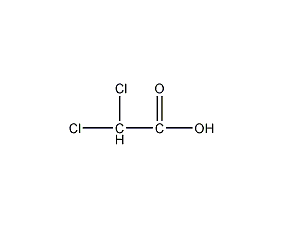
Structural formula
| Business number | 01PY |
|---|---|
| Molecular formula | C2H2O2Cl2 |
| Molecular weight | 129 |
| label |
dichloroacetic acid, Bichloroethanoic acid, corrosive, Multifunctional solvents, aliphatic compounds |
Numbering system
CAS number:79-43-6
MDL number:MFCD00004223
EINECS number:201-207-0
RTECS number:AG6125000
BRN number:1098596
PubChem number:24862522
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): 9~11[2]
3. Boiling point (℃) : 194[3]
4. Relative density (water = 1): 1.56[4]
5. Relative Vapor density (air=1): 4.45[5]
6. Saturated vapor pressure (kPa): 0.13 (44℃)[6]
7. Octanol/water partition coefficient: 0.92[7]
8. Flash point (℃): 110[8]
9. Explosion upper limit (%): 43.3[9]
10. Explosion lower limit (%): 11.9[10 ]
11. Solubility: soluble in water, ethanol, and ether. [11]
12. Heat of formation (KJ/mol, liquid): 503.3
13. Conductivity (S/m, 0ºC): 4 ×10-8
14. Conductivity (S/m, 25ºC): 7×10-8
15 .Refractive index at room temperature (n20): 1.4658
16. Relative density (25℃, 4℃): 1.530235
17. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -496.3
18. Liquid phase standard hot melt (J·mol -1·K-1): 172.7
Toxicological data
1. Acute toxicity: rat caliber LD50: 2820mg/kg; rabbit skin LD50: 510 uL/kg;
2. Neurotoxicity: rabbit skin test: 2mg/24HREACTION;
3. Other multiple dose toxicity data:
Rat caliber TDL0: 3195 mg/kg/90D-C; caliber TDL0: 7100 mg/kg/10W-C;
4. Chronic toxicity/carcinogenicity: rat caliber TDL0: 100 mg/kg/2Y-C;
5. Teratogenicity: mice: 1800mg/kg/9D; mice: 645 mg /kg;
6. It is of low toxicity. Strongly corrosive and irritating to skin and mucous membranes. It produces toxic chloride vapor when decomposed by heat. Reacts with water or water vapor to produce toxic corrosive gases. Inhalation of saturated vapor in rats for 8 hours did not cause death, but severe skin and eye damage occurred. Dichloroacetic acid has a strong exfoliating effect.
7. Acute toxicity[12] LD50: 2820mg/kg (rat oral); 510mg/kg (Rabbit transdermal)
8. Irritation[13] Rabbit transdermal: 2mg (24h), Severe irritation.
9. Mutagenicity[14] Micronucleus test: mice oral 1800mg/kg (9d) (continuous). DNA damage: mouse oral administration 645mg/kg. Cytogenetic analysis: mouse lymphocytes 600mg/L. mammalian somatic cellsChange: Mouse lymphocytes 400mg/L
10. Teratogenicity[15] After pregnancy in rats The lowest toxic dose (TDLo) of 4g/kg after oral exposure for 6 to 15 days can cause developmental abnormalities of the cardiovascular system. The lowest toxic dose (TDLo) of rats exposed 6 to 15 days after pregnancy was 14g/kg, which caused developmental malformations of the genitourinary system.
11. Carcinogenicity[16] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals .
Ecological data
1. Ecotoxicity[17] LC50: 0.07~0.12mg/L (48h) (fish); 0.025~0.12mg /L (48h) (invertebrates)
2. Biodegradability No data available
3. Non-biodegradability[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 22d (theoretical).
Molecular structure data
1. Molar refractive index: 22.53
2. Molar volume (cm3/mol): 79.3
3. Isotonic specific volume (90.2K ): 207.7
4. Surface tension (dyne/cm): 47.0
5. Polarizability (10-24cm3): 8.93
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 60.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: Stronger acidity than chloroacetic acid (Ka=5×10-2). It is relatively stable to hydrolysis, but the chlorine atom is relatively easy to be replaced. Like chloroacetic acid, various derivatives can be generated.
2. Stability[19] Stability
3. Incompatible substances[20] Strong oxidizing agent, strong alkali, strong reducing agent
4. Conditions to avoid contact [21] Heating p>
5. Polymerization hazard[22] No polymerization
6. Decomposition products[23] sup> Hydrogen chloride
Storage method
Storage Precautions[24] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and reducing agents, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Acetic acid chlorination mother liquor recovery method: chlorine acetic acid to obtain chloroacetic acid mother liquor. The chloroacetic acid mother liquor undergoes chlorination reaction under the catalysis of sulfur and is obtained by distillation.
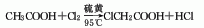
2. Trichloroacetaldehyde method Trichloroacetaldehyde is obtained by cyanation, dehydrochlorination and hydrolysis.
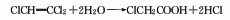
3. The acetic acid method consists of acetic acid in It is obtained by chlorination under iodine catalysis.
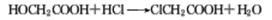
Refining method: Chlorination with acetic acid The crude product obtained is esterified with lower alcohol, and the pure product can be obtained by saponifying the ester after fractionation. If a mixture of dichloroacetic acid and trichloroacetic acid needs to be separated, hydrolysis can be used. Trichloroacetic acid generates chloroform and carbon dioxide after hydrolysis, and then the dichloroacetic acid is extracted with a water-insoluble solvent such as ether. In addition, it can also be refined by benzene recrystallization.
Purpose
1. Used as an organic synthesis intermediate for the preparation of methyl dichloroacetate (chloramphenicol intermediate), pharmaceutical allantoin and cationic dyes, etc. Also used as a corrosive agent.
2. Used in organic synthesis and drug manufacturing. [25]

 微信扫一扫打赏
微信扫一扫打赏

