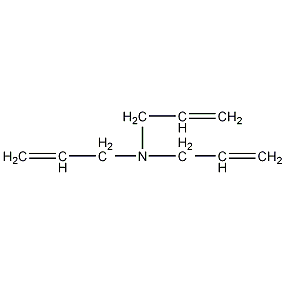
Structural formula
| Business number | 02MC |
|---|---|
| Molecular formula | C9H15N |
| Molecular weight | 137.22 |
| label |
Triallylamine, N235, LTS propylamine, N235, Extracting agent |
Numbering system
CAS number:102-70-5
MDL number:MFCD00026093
EINECS number:203-048-2
RTECS number:XX5950000
BRN number:None
PubChem number:24900211
Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 25℃): 0.79
3. Relative vapor density (g/mL, air=1 ): 4.73
4. Melting point (ºC): -70
5. Boiling point (ºC, normal pressure): 150-151
6. Refractive index : 1.451
7 Flash point (ºC): 39.4
8. Vapor pressure (mmHg, 80ºC): 90
9. Solubility: Slightly soluble in Water, soluble in ethanol, ether, acetone, benzene.
Toxicological data
1, skin/ Eye irritation: Start irritation test: rabbit skin contact, 10mg/24HREACTION SEVERITY, strong response; Flush test: Rabbit eye contact, 50 mg/20SREACTION SEVERITY, slight reaction;2. Acute toxicity: Human inhalationTCLo:13ppm/5M; Rat OralLD50:1030 mg/kg; span> Rat inhalationLC50:554ppm/8H ; Mouse oral LD50: 492mg/kg; “>MousePeritoneal cavityLD50:187 mg/kg -family:宋体;color:#000000;FONT-SIZE: 9pt”>Rabbit skin contactLD50: 400μL/kg; Mammalian InhalationLC50:2800 mg/m3; Mammalian route unknown LD50:620mg/kg;3. Other multiple dose toxicity: Rat inhalation TCLo:200ppm/7H/50D-I
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 46.64
2. Molar volume (cm3/mol): 169.6
3. Isotonic specific volume (90.2K): 382.9
4. Surface tension (dyne/cm): 25.9
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 18.49
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 3.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 92.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, strong acids, and strong alkali. It is a light yellow transparent liquid at room temperature. It is insoluble in water, but soluble in organic solvents such as ethanol and ether, and has a pungent odor. The product is alkaline, and is more alkaline than ammonia, and can react with acid to form salts. It plays a role in liquid ion exchange when extracting and concentrating cations in acidic aqueous solutions. It can form organic solvent complex salts with various metals, such as uranium, vanadium, chromium, calcium, platinum, etc.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
C8-10 fatty alcohol is used as raw material, reacts with ammonia in the presence of a catalyst, and then undergoes a condensation reaction with fatty alcohol to obtain the product.
Purpose
Mainly used as an extraction agent for uranium and rare metals; it can also be used to treat organic wastewater and produce additives and metal resists for petroleum products, quaternary ammonium compounds and their derivatives. It is used in organic synthesis and resin modification, and also serves as a cross-linking agent for high absorbents and an intermediate for ion exchange resins.
-FAMILY: 宋体”>3. Other multi-dose toxicity:Rat inhalationTCLo:200ppm/7H/50D-I
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 46.64
2. Molar volume (cm3/mol): 169.6
3. Isotonic specific volume (90.2K): 382.9
4. Surface tension (dyne/cm): 25.9
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 18.49
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 3.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 92.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, strong acids, and strong alkali. It is a light yellow transparent liquid at room temperature. It is insoluble in water, but soluble in organic solvents such as ethanol and ether, and has a pungent odor. The product is alkaline, and is more alkaline than ammonia, and can react with acid to form salts. It plays a role in liquid ion exchange when extracting and concentrating cations in acidic aqueous solutions. It can form organic solvent complex salts with various metals, such as uranium, vanadium, chromium, calcium, platinum, etc.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
C8-10 fatty alcohol is used as raw material, reacts with ammonia in the presence of a catalyst, and then undergoes a condensation reaction with fatty alcohol to obtain the product.
Purpose
Mainly used as an extraction agent for uranium and rare metals; it can also be used to treat organic wastewater and produce additives and metal resists for petroleum products, quaternary ammonium compounds and their derivatives. It is used in organic synthesis and resin modification, and also serves as a cross-linking agent for high absorbents and an intermediate for ion exchange resins.

 微信扫一扫打赏
微信扫一扫打赏

