
Structural formula
| Business number | 030C |
|---|---|
| Molecular formula | C4H4O |
| Molecular weight | 68.07 |
| label |
Monoxadiene pentacyclic ring, Oxacene, Oxol, Divinylene Oxide, heterocyclic compounds, intermediates, synthetic raw materials |
Numbering system
CAS number:110-00-9
MDL number:MFCD00003222
EINECS number:214-757-1
RTECS number:LT8524000
BRN number:103221
PubChem ID:None
Physical property data
1. Properties: colorless liquid with mild fragrance. [13]
2. Melting point (℃): -85.6[14]
3. Boiling point (℃): 31.4[15]
4. Relative density (water = 1): 0.94[16]
5. Relative vapor Density (air=1): 2.35[17]
6. Saturated vapor pressure (kPa): 65.6 (20℃)[18]
7. Heat of combustion (kJ/mol): -2090.4[19]
8. Critical pressure (MPa): 5.32[20]
9. Octanol/water partition coefficient: 1.34[21]
10. Flash point (℃): -35 (CC )[22]
11. Ignition temperature (℃): 390[23]
12. Explosion upper limit ( %): 14.3[24]
13. Lower explosion limit (%): 2.3[25]
14. Dissolution Properties: Insoluble in water, soluble in acetone, benzene, easily soluble in most organic solvents such as ethanol and ether. [26]
15. Refractive index (n20ºC): 1.4214
16. Heat of evaporation (J/mol, 31.2ºC): 399.8
17. Heat of formation (KJ/mol): 62.0
18. Relative density (20℃, 4℃): 0.9514
19. Critical density (g·cm -3): 0.312
20. Critical volume (cm3·mol-1): 218
21. Critical compression factor: 0.294
22. Eccentricity factor: 0.200
23. Solubility parameter (J·cm-3) 0.5: 18.541
24. van der Waals area (cm2·mol-1): 4.920×109
25. van der Waals volume (cm3·mol-1): 37.580
26. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2110.95
27. Gas phase standard claimed heat (enthalpy) (kJ·mol -1): -34.73
28. Gas phase standard entropy (J·mol-1·K-1): 267.25
29. Gas phase standard formation free energy (kJ·mol-1): 0.9
30. Gas phase standard hot melt (J·mol– 1·K-1): 65.40
31. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1) : -2083.30
32. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -62.38
33. Liquid phase standard entropy (J·mol-1·K-1): 176.95
34. Liquid phase standard formation free energy (kJ·mol– 1): 0.17
35. Liquid phase standard hot melt (J·mol-1·K-1): 114.56
Toxicological data
1. Urgent��A good way to synthesize polyene compounds (Formula 8)[9].
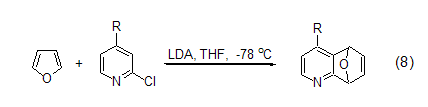
The reaction between furan and dipolar substances gives 8-Oxo-bicyclo[3.2.1]dec-6-en-3-one (Formula 9)[10]. These [4+3] cycloaddition products can be easily converted into cycloheptanone, cycloheptantrienone and substituted cycloheptane systems, or substituted tetrahydrofuran compounds.
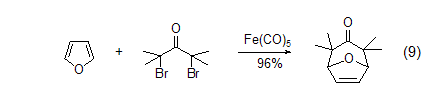
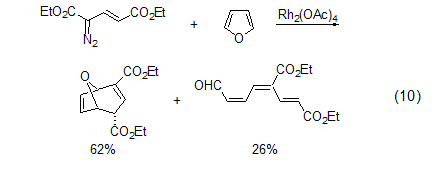
Carbene reaction
strong> Including the reaction of carbene addition to generate 2-oxo-bicyclo[3.1.0]hex-3-ene, etc. The reaction of furan and vinylcarbene will give two products (Formula 10)[11,12].
2. Stability [33] Stable
3. Incompatible materials[34] Strong oxidants, acids
4. Conditions to avoid contact[35] Air
5. Polymerization hazard[36] No aggregation
Storage method
Storage Precautions[37] Usually products contain polymerization inhibitors. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29°C. Keep away from light. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants and acids, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Furfural is oxidized to obtain 2-furancarboxylic acid, and after decarboxylation, furan is obtained. When 2-furancarboxylic acid is heated to 200-205°C (around the boiling point), it decomposes into furan and carbon dioxide. During the reaction, the sublimated 2-furancarboxylic acid is returned to the reactor, the distilled furan is re-distilled, and the 31-34°C fraction is collected to obtain a relatively pure finished product. The yield is about 75%. Industrially, furfural can be directly decarbonylated. The catalysts used in the reaction include aluminum silicate, metal oxides or hydroxides, and mixtures of alloys or metals, such as a mixture of zinc chromite and manganese. The reaction temperature is about 400°C, and the yield is 90%. In large-scale production, the yield can reach 74%.
Refining method: wash with 5% potassium hydroxide aqueous solution, dry with anhydrous calcium sulfate or anhydrous sodium sulfate, and distill in the presence of potassium hydroxide or metallic sodium before use. If there is peroxide, it can be removed by washing with sodium bisulfate and weakly acidic ferrous sulfate aqueous solution.
Purpose
1. Used to prepare pyrrole, thiophene, tetrahydrofuran, etc. Furan is etherified and reduced to obtain 2,5-dimethoxydihydrofuran, which is hydrolyzed to produce 2-hydroxy-1,4-butanedialdehyde, which can be used for the production of anisodamine by the synthetic method; when furan is etherified, When reduced and then catalytically hydrogenated to obtain 2,5-dimethoxytetrahydrofuran, it is hydrolyzed to form succinic aldehyde, which is the raw material for the synthesis of another alkaloid, atropine. Furan is also used to produce the anti-inflammatory drug sodium toluyl acetate, which consumes 4.75t of furan per ton of this drug.
2. Used in organic synthesis or as a solvent. [38]

 微信扫一扫打赏
微信扫一扫打赏

