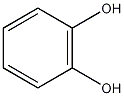
Structural formula
| Business number | 03CV |
|---|---|
| Molecular formula | C6H6O2 |
| Molecular weight | 110 |
| label |
1,2-dihydroxybenzene, pyrocatechol, 1,2-Benzodiphenol, catechol, 1,2-Benzenediol (pyrocatechol), 1,2-benzenediol[qr], 1,2-Dihydroxybenzene, pyrocatechol, Pesticide intermediates; phenols, aromatic alcohols and their derivatives |
Numbering system
CAS number:120-80-9
MDL number:MFCD00002188
EINECS number:204-427-5
RTECS number:UX1050000
BRN number:471401
PubChem number:24278346
Physical property data
1. Properties: Colorless crystal, changes color when exposed to light or exposed to air, and can sublimate. [1]
2. pH value: <7 (1% solution) [2]
3. Melting point ( ℃): 105[3]
4. Boiling point (℃): 245~246[4]
5. Relative density (water=1): 1.34[5]
6. Relative vapor density (air=1): 3.79[6]
7. Saturated vapor pressure (kPa): 0.0039 (20℃)[7]
8. Heat of combustion (kJ/mol): -2854.9[8]
9. Critical pressure (MPa): 749[9]
10. Octanol/water partition coefficient: 0.88[10]
11. Flash point (℃): 127 (CC) [11]
12. Ignition temperature (℃): 510[12]
13. Explosion limit (%): 9.8[13]
14. Lower explosion limit (%): 1.6[14]
15. Solubility: soluble in water, ethanol, ether, benzene, chloroform, and alkali. [15]
16. Relative density (25℃, 4℃): 1.137131
17. Relative density ( 20℃, 4℃): 1.149322
18. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): – 2867.7
19. Crystal phase standard claim heat (enthalpy) (kJ·mol-1): -351.0
20. Gas phase standard combustion heat (enthalpy) )(kJ·mol-1): -2946.7
21. Gas phase standard claims heat (enthalpy) (kJ·mol-1): – 272.0
22. Gas phase standard entropy (J·mol-1·K-1): 333.75
23. Gas phase Standard free energy of formation (kJ·mol-1): -183.1
24. Gas phase standard hot melt (J·mol-1·K-1):120.09
Toxicological data
1. Acute toxicity[16] LD50: 260mg/kg (rat oral); 800mg/kg (rabbit dermal )
2. Irritation No information available
3. Mutagenicity[17] Microbial mutagenicity: Salmonella typhimurium 15μmol/dish. DNA inhibition: human HeLa cells 10 μmol/L. Sister chromatid exchange: human lymphocyte μmol/L. Cytogenetic analysis: hamster lung 60 μmol/L (2h).Pour chlorine gas into the material and control the temperature at 24~28℃
Whentherelativedensityofthemixedsolutionreaches0.954,stoppassingchlorineanddischargethehydrogenchlorideunderreducedpressure.Aftersteamingoutexcessbenzeneat21332Paand125℃,coolitto60℃,andthenperformvacuumdistillationatapressureof2666~3333Pa.Collectthemiddlefraction(75℃)aso-chlorophenol.Lowboilers(75℃)canberecycled.Afterthepreparedo-chlorophenolismixedwithcoppersulfateandsodiumhydroxideinanappropriateproportion,itispassedintoatubularreactorthathasbeenpreheatedto230-2240°Cforreaction:
Control the reaction temperature to 180~190℃ and the reaction residence time to 50~60min. The reaction liquid flowing out after the reaction enters a container containing hydrochloric acid for neutralization. Control the ph value of neutralization to 3~ 3.5:
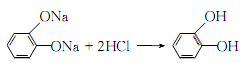
Afterwards, it is decolorized with activated carbon, filtered, and the filtrate is extracted with isopropyl acetate in countercurrent flow. The extract is distilled under normal pressure and under reduced pressure. Catechol can be obtained.
Purpose
1. As an important pharmaceutical intermediate, it is used to manufacture berberine and isoproterenol, etc. It can also be used to produce 4-tert-butylcatechol as a polymerization inhibitor for styrene, butadiene and vinyl chloride. Or used to make antioxidants; developers; bactericides; rubber additives; electroplating additives; special inks; light stabilizers; dyes; spices, etc.
2. Used to synthesize vanillin, ethyl vanillin, piperonal, etc. In the pharmaceutical industry, it is used for alloberberine and isoproterenol. It can also be used in dyes, pesticides, photosensitive materials, electroplating materials, oxygen disruptors, light stabilizers, preservatives and accelerators.
3. An important intermediate in drug synthesis, used to produce berberine and isoproterenol, etc., and can also be used to produce 4-tert-butylcatechol. It is also used as a polymerization inhibitor for styrene, butadiene and vinyl chloride. It is also used in the manufacture of antioxidants, developers, bactericides, preservatives, accelerators, electroplating additives, special inks, light stabilizers, dyes, spices, etc.
4. Used in photography, dyes, antioxidants, light stabilizers, and important pharmaceutical intermediates. [28]

 微信扫一扫打赏
微信扫一扫打赏

