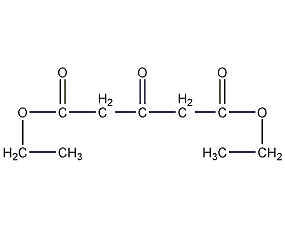
Structural formula
| Business number | 02RT |
|---|---|
| Molecular formula | C9H14O5 |
| Molecular weight | 202.20 |
| label |
Diethyl 3-oxoglutarate, Diethyl 3-oxoglutarate |
Numbering system
CAS number:105-50-0
MDL number:MFCD00009200
EINECS number:203-302-2
RTECS number:None
BRN number:640146
PubChem number:24850124
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25℃): 1.112
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 250
6. Boiling point (ºC, 10mmHg): 134-135
7. Refractive index: 1.438-1.442
8. Flash point (ºC): 86
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor pressure (kPa , ℃): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15 . Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V ): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, soluble in alcohol, ether and benzene.
Toxicological data
None
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 47.32
2. Molar volume (cm3/mol): 181.8
3. Isotonic specific volume (90.2K ): 444.7
4. Surface tension (dyne/cm): 36.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 18.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: 5
6. Topological molecule polar surface area 69.7
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 199
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Covalent bond unitQuantity:1
Properties and stability
Avoid contact with oxidants.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants and strong alkali, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of 1,3-acetone dicarboxylic acid and absolute ethanol.
2. Preparation method:
Add 700mL of absolute ethanol into the reaction bottle, and pass in dry hydrogen chloride gas under cooling (can be prepared by the reaction of sodium chloride and sulfuric acid, and treated with concentrated sulfuric acid Dry) until 130-150g of hydrogen chloride ① is absorbed, add 460g (3.15mol) of acetone dicarboxylic acid (2) in batches while stirring. Slowly heat to 45°C until the acetone dicarboxylic acid is completely dissolved. Cool to room temperature and stir the reaction for 12 hours. Pour the reactant into 1.4L water and separate the organic layer. The aqueous layer was extracted with chloroform (500mL×2). The organic layers were combined, washed with saturated sodium bicarbonate aqueous solution, and dried over anhydrous sodium sulfate. The solvent was evaporated and then distilled under reduced pressure. The fraction at 131-136°C/1.2-1.3kPa was collected to obtain 295g of diethyl acetone dicarboxylate (1) with a yield of 46.4%. Note: ① Acetone dicarboxylic acid is unstable, so it is best not to use concentrated sulfuric acid as a catalyst for the esterification reaction. [1]
Purpose
Organic synthesis intermediates.

 微信扫一扫打赏
微信扫一扫打赏

