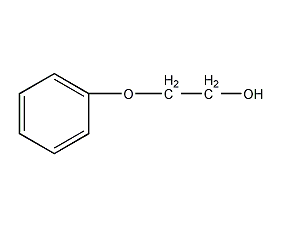
Structural formula
| Business number | 03FW |
|---|---|
| Molecular formula | C8H10O2 |
| Molecular weight | 138.17 |
| label |
Ethylene glycol phenyl ether, Ethylene glycol phenyl ether, phenyl cellosolve, Ethylene glycol monophenyl ether, 1-Hydroxy-2-phenoxyethane, β-Hydroxyethyl phenyl ether, fragrance retainer, High boiling point organic solvents, alcohol solvent |
Numbering system
CAS number:122-99-6
MDL number:MFCD00002857
EINECS number:204-589-7
RTECS number:KM0350000
BRN number:1364011
PubChem number:24898156
Physical property data
1. Properties: colorless oily liquid
2. Boiling point (ºC): 245.2
3. Melting point (ºC): 14
4. Relative density (d204) : 1.1094
5. Refractive index (n20D): 1.543
6. Viscosity (mPa·s, 20ºC): 30.5
7. Flash point (ºC): 121
8. Heat of evaporation (KJ/mol): 39.8
9. Specific heat capacity (KJ/(kg·K), 15ºC, constant pressure): 2.03
10. Vapor pressure (kPa, 20ºC): 0.004
11. Vapor pressure (kPa, 118ºC): 1.333
12. Vapor pressure (kPa, 157ºC): 6.666
13. Body expansion coefficient (K-1): 0.00077
14. Solubility: 25% dissolved in water at 20°C; water dissolves in ethylene glycol monophenyl ether Dissolved in 10.6%. Soluble in alcohol, ether and sodium hydroxide aqueous solution. Can dissolve grease, natural resin, alkyd resin, cellulose acetate, ethyl cellulose, nitrocellulose and polyvinyl acetate, etc. Miscible with glycerol, propylene glycol ethanol, ether and benzene.
15. Relative density (20℃, 4℃): 1.1082
16. Refractive index at room temperature (n25): 1.5278
Toxicological data
1. Skin/eye irritation: Rabbit skin standard Drez eye dye test: 500mg/24H is slightly irritating to the skin.
Rabbit Eye Standard Draz Dye Eye Experiment: 6mg has a medium -intensity stimulus on the eyes.
Rabbit Eye Standard Drez Dye Eye Experiment: 250ug/24h has a serious stimulating effect on the eyes.
2. Acute toxicity: rat oral LD50: 1260mg/kg; rat skin LD50: 14422mg/kg; LCLO: 933mg/kg;
The mice passed through the peritoneal membrane LC50: 490mg/kg; the rabbit skin LD50: 5ml/kg;
Mouse port TDLO: 182gm/kg/13W-I; Rat Outlet TDLO: 17500mg/kg/14d-i
Rat inhale TDLO: 15mg/m 3 // 9W-I; Rabbit oral TDLO: 3gm/kg/10D-I;
Rabbit skin TDLO: 14gm/kg/14D-I 4. Mutagenicity: E. coli DNA Inhibition test system: 2000ppm; E. coli mutation test system: 2000ppm
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 39.09
2. Molar volume (cm3/mol): 127.4
3. Isotonic specific volume (90.2K ): 320.4
4. Surface tension (dyne/cm): 39.9
5. Polarizability (10-24cm3): 15.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 77.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is not easily absorbed by the skin, so be careful to protect your eyes when using it. Chemical properties have the chemical properties of primary alcohols. When heated together with zinc chloride, a ring-closing reaction occurs to form oxaindane.
2. Found in burley tobacco leaves.
Storage method
Store in a cool, dry place.
Synthesis method
1. Obtained from the addition of phenol and ethylene oxide. Sodium acetate or sodium hydroxide is used as a catalyst, the reaction temperature is 200°C, and the pressure is 0.2~0.25MPa.
2. Refining method: Impurities that are easy to contain include the addition products of ethylene oxide and ethylene glycol. It is generally refined by fractional distillation.
3. Tobacco: BU, 14.
Purpose
1. Ethylene glycol phenyl ether (KL-EPH) is a typical high boiling point organic solvent, which has been widely used in Europe and the United States as early as the 1970s. It is a colorless and transparent liquid with a slight rose fragrance, low volatility and high boiling point. Because it is miscible with many organic solvents and has strong permeability, it is more comprehensive than isophorone (commonly known as 783), DBE and benzyl alcohol. Outstanding performance, can be used as a full-performance replacement. KL-EPH has excellent solubility in various resins such as acrylic resin, nitrocellulose, ethylcellulose, epoxy resin, alkyd resin, phenoxy resin, etc., and is miscible with alcohol and ether, commonly known as ” Universal Solvent”. It is used as a solvent for impressions and engraving inks, as a binder and as a flavor retaining agent for soaps and cosmetics. It is also used as a solvent for cellulose acetate, resins, dyes, and synthetic plasticizers, fungicides, drugs, etc.
2.The maximum allowable content (mass fraction) in cosmetics is 1.1%. It is often used in combination with parabens, dehydroacetic acid and sorbic acid, and propylene glycol is generally added to increase its solubility. It is used in combination with quaternary ammonium salts as a fungicide and also as an insecticide.
3. Because the aroma is very weak, it is suitable to be used as a fixative or solvent in daily chemical fragrances.

 微信扫一扫打赏
微信扫一扫打赏

