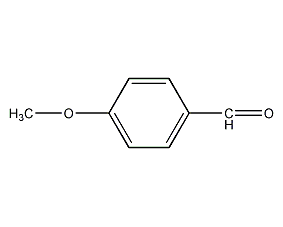
Structural formula
| Business number | 03G6 |
|---|---|
| Molecular formula | C8H8O2 |
| Molecular weight | 136.15 |
| label |
anisaldehyde, 4-Methoxybenzaldehyde, Anisic aldehyde, Aubepine, p-Methoxybenzaldehyde, fragrance auxiliary, modifier, Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:123-11-5
MDL number:MFCD00003385
EINECS number:204-602-6
RTECS number:BZ2625000
BRN number:471382
PubChem number:24891369
Physical property data
1. Properties: colorless oily liquid
2. Relative density: 1.119 g/cm3
3. Melting point (℃): 0
4. Boiling point (℃): 248
5. Flash point (℃): 108
6. Fire point (℃, open cup): 117.8
7. Solubility: Slightly soluble in water, miscible with ethanol and ether, easily soluble in acetone and chloroform, and soluble in benzene.
8. Relative density (20℃, 4℃): 1.119115
9. Relative density (25℃, 4℃): 1.029131
10. Normal temperature refractive index (n20): 1.5731
11. Gas phase standard combustion heat (enthalpy) (kJ· mol-1): -4088.7
12. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -202.7
13. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -4024.2
14. Liquid phase standard claimed heat (enthalpy) (kJ ·mol-1):-267.2
Toxicological data
1. Acute toxicity: Rat oral LD50: 1510mg/kg
Rat intravenous injection LD50: 680mg/kg
Rabbit skin LD50: >5gm/kg
Dolphin and pig oral LD50: 1260mg/kg
2. Other multiple dose toxicity: rat oral TDLO: 55220ug/kg/30D-I
3 , Mutagenic toxicity: Human lymphocyte double chromosome exchange test system: 1mmol/L
Mouse lymphocyte DNA damage test system: 7020umoL/L
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 39.68
2. Molar volume (cm3/mol): 125.1
3. Isotonic specific volume (90.2K ): 309.0
4. Surface tension (dyne/cm): 37.2
5. Polarizability (10-24cm3): 15.73
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 104
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1.The gas forms an explosive mixture with air. Protective glasses, protective clothing, and protective gloves should be worn.
2. Exist in tobacco leaves and smoke.
3. Naturally found in anise oil, cumin oil, star anise oil, dill oil, acacia oil, corn oil and other essential oils .
4. It is not stable to light and is easily oxidized and discolored in the air to form anisic acid.
5. p-Methoxybenzaldehyde can be used to protect diols, dithiols, amines, hydroxylamines and diamines.
Protection of diols Para-methoxybenzaldehyde can easily be reacted with diols and aldehydes to form acetal. The catalyst used can be hydrochloric acid or chlorine. Zinc chloride can also be oxidized using other methods such as iodine catalysis[1], polyaniline-based sulfuric acid catalysis[2] (see formula 1), indium trichloride Catalysis[3], bismuth nitrate catalysis[4], etc. p-Methoxybenzaldehyde reacts with L-cysteine to obtain thiazole derivatives (Formula 2)[5].

Reacts with amino groups p-Methoxybenzaldehyde can react with amino groups to form Schiff base, which can be reduced by NaBH4 to form a secondary amine (formula 3)[6].

Form ethylene oxide Alkane derivatives p-Methoxybenzaldehyde can react with sulfur ylide to form ethylene oxide derivatives (Formula 4)[7], or it can react with diazo compounds to obtain this Derivatives (Formula 5)[8]. By reacting with ethylene oxide derivatives, the ring can also be expanded to obtain furan ring derivatives (Formula 6)[9].

Diacylation reaction Under the catalysis of tetrabutylammonium bromide (TBATB), p-methoxybenzaldehyde can react with acid anhydride to form a diacyl product (Formula 7)[10].

allylation Reaction Due to the strong electron donating effect of the para-methoxy group, p-methoxybenzaldehyde reacts with allyltrimethylsilane under the catalysis of bismuth trifluorosulfonate to obtain the diallylated product (Formula 8)[11].

Storage method
200kg plastic drum, 5-25kg polyethylene or polypropylene plastic drum, plus cardboard drum. Should be stored away from light.
Synthesis method
Production method: Mainly using chloromethylation method. Anisole is used as raw material, and in the presence of formaldehyde and hydrogen chloride, a chloromethylation reaction is performed to generate p-methoxybenzyl chloride, which is then salted with methenamine, and then hydrolyzed with hydrochloric acid, separated, washed with water, and decompressed Distilled.

Purpose
1. Aldehyde synthetic fragrances. Mainly used as the fragrance base of hawthorn, sunflower, lilac and other flavors. It is also a blending spice for fresh mowing grass, silvery white acacia flowers, acacia, perfume and other essences. It is also a flavor auxiliary for lily of the valley essence and a modifier for osmanthus essence.
2. Widely used in daily chemical flavors and food flavor formulas. As a medium-flavor spice, it is widely used in the preparation of floral flavors; it is also used in medicine, food and daily chemical industries; as an excellent brightener for cyanide-free zinc plating DE additives.

 微信扫一扫打赏
微信扫一扫打赏

