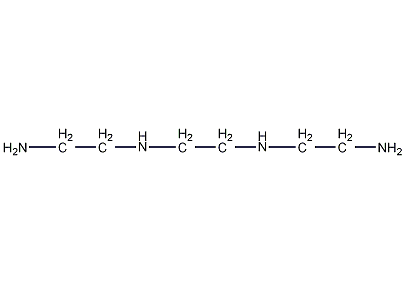
Structural formula
| Business number | 0359 |
|---|---|
| Molecular formula | C6H18N4 |
| Molecular weight | 146.23 |
| label |
Triethylenetetramine, Triethylenetetramine, Triethylenediamine diamine, Triethylenetetraaminehexaacetic, N,N’-Bis(2-ammonia base B)ethylene diamine, Hardener, rubber vulcanization accelerator, stabilizer, Surfactant, emulsifier, Lubricant additives, gas purifier, Cyanide-free electroplating diffusion agent, brightener, Detergent |
Numbering system
CAS number:112-24-3
MDL number:MFCD00008169
EINECS number:203-950-6
RTECS number:YE6650000
BRN number:605448
PubChem number:24889370
Physical property data
1. Properties: colorless or slightly yellow viscous liquid. [1]
2. pH value: 14[2]
3. Melting point (℃): 12[3]
4. Boiling point (℃): 267[4]
5. Relative density (water=1): 0.99[5]
6. Relative vapor density (air=1): 5.04[6]
7. Critical Pressure (MPa): 3.17[7]
8. Octanol/water partition coefficient: -2.65[8]
9. Flash point (℃): 135 (CC) [9]
10. Ignition temperature (℃): 338[10]
11. Explosion upper limit (%): 6.5[11]
12. Explosion lower limit (%): 1[12] sup>
13. Solubility: Miscible with water, slightly soluble in ether, soluble in ethanol and acid. [13]
Toxicological data
1. Acute toxicity[14] LD50: 4340mg/kg (rat oral); 805mg/kg (rabbit dermal)
2. Irritation[15]
Rabbit transdermal: 5mg (24h), severe irritation.
Rabbit eye: 49mg, severe irritation.
3. Subacute and chronic toxicity [16] Long-term skin contact can cause serious damage to ulcers and necrosis.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[17] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: 44.10
2. Molar volume (cm3/mol): 152.5
3. Isotonic specific volume (90.2K): 384.8
4. Surface tension (dyne/cm): 40.4
5. Polarizability (10-24cm3): 17.48
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -2.5
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: none
6. Topological molecule polar surface area 76.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 49.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. A yellow liquid with strong alkalinity and medium viscosity, its volatility is lower than diethylenetriamine. But other properties are similar. Soluble in water and ethanol, slightly soluble in ether. Aqueous solution is a strong base that can react with acidic oxides, acid anhydrides, aldehydes, ketones, and halides. Can corrode metals such as aluminum, zinc, copper and their alloys.
2. Stability[18] Stable
3. Incompatible substances[19] Acids, acid chlorides, acid anhydrides, strong oxidants, chloroform
4. Polymerization hazards [20] No polymerization
5. Decomposition products[21] Ammonia, amine
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
The production method is dichloroethane amination method. Send 1,2-dichloroethane and ammonia water into a tubular reactor to perform a hot-pressure ammoniation reaction at a temperature of 150-250°C and a pressure of 392.3kPa. The reaction solution is neutralized with alkali to obtain mixed free amines, which are concentrated while removing sodium chloride. The crude product is then distilled under reduced pressure and the fraction between 195-215°C is intercepted to obtain the finished product. This method co-produces ethylenediamine, diethylenetriamine, tetraethylenepentamine and polyethylenepolyamine at the same time. It can be obtained by controlling the temperature of the distillation tower to distill the amine mixture and intercepting different fractions for separation. .
Purpose
1. Used as complexing reagent, dehydrating agent for alkaline gas, dye intermediate, solvent for epoxy resin, etc. Used as room temperature curing agent for epoxy resin, reference dosage 10-12 parts by mass, curing conditions intermediate and solvents. This product can also be used as rubber vulcanization accelerator and stabilizer, surfactant, emulsifier, lubricant additive, gas purifier, cyanide-free electroplating diffusing agent, brightener, fabric finishing agent, ion exchanger resin, and polyamide resin. synthetic raw materials. It can also be used as vulcanizing agent for fluorine rubber.
2. Used as a room temperature curing agent for epoxy resin, the reference dosage is 10 to 12 parts by mass, and the curing conditions are room temperature/2d or 100℃/30min. The thermal deformation temperature of the cured product is 98~124℃. Also used as organic synthesis intermediates and solvents. Used in the manufacture of polyamide resins, ion exchange resins, surfactants, lubricating oil additives, gas purifiers, etc. It is also used as metal chelating agent, cyanide-free electroplating diffusion agent, rubber additive, brightener, detergent, dispersant, etc.
3. Used as complexing reagent, dehydrating agent for alkaline gas, dye intermediate, solvent for epoxy resin, etc. [23]

 微信扫一扫打赏
微信扫一扫打赏

