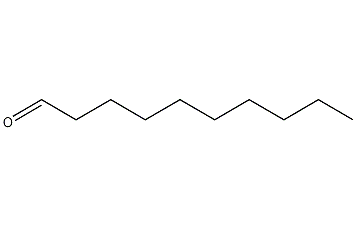
Structural formula
| Business number | 035F |
|---|---|
| Molecular formula | C10H20O |
| Molecular weight | 156.27 |
| label |
decanal, Decacarbonic aldehyde, caprylic aldehyde, n-Decanal, Decyl aldehyde, n-Decanal, n-Capnic aldehyde, Decanal, food additives, Flavor enhancer |
Numbering system
CAS number:112-31-2
MDL number:MFCD00007031
EINECS number:203-957-4
RTECS number:HD6000000
BRN number:1362530
PubChem number:24894127
Physical property data
1. Properties: Colorless to light yellow liquid with a strong aldehyde aroma. When diluted, it has the aroma of sweet orange and tangerine, and has a grease smell.
2. Density (g/mL, 25℃): 0.830
3. Relative density (20℃, 4℃): 0.8250
4. Melting point (ºC): -4
5. Boiling point (ºC, normal pressure): 208~209
6. Boiling point (ºC, 0.93KPa): 81
7. Relative density (25℃, 4℃): 0.8211
8. Flash point (ºC): 83
9. Refractive index at room temperature (n20): 1.4272
10. Refractive index at room temperature (n25): 1.4251
11. Vapor pressure (mmHg, 70ºC):
12. Saturated vapor pressure (kPa, ºC):
13. Critical temperature (ºC): 400.85
14. Critical pressure (MPa): 2.60
15. Critical density (g·cm-3): 0.261
16. Critical volume (cm3·mol -1): 599
17. Critical compression factor: 0.278
18. Eccentricity factor: 0.642
19. Solubility: insoluble in Water and glycerin, soluble in fatty oil, volatile oil, mineral oil and 80% alcohol.
20. Solubility parameter (J·cm-3)0.5: 17.834
21. van der Waals area ( cm2·mol-1): 1.529×1010
22. van der Waals volume (cm3·mol-1): 110.165
23. Liquid phase standard hot melt (J·mol-1·K-1): 349.2
Toxicological data
1. Irritation: Rabbit percutaneous open irritation test: 14372ug/24H severe irritation.
Rabbit transdermal Drez eye dye test: 500mg/24H Mild irritation.
2. Acute toxicity: Rat oral LD5O: 3730ul/kg
Mouse oral LC5O: >41750mg/kg
�Subcutaneous transdermal LD5O: 5040ul/kg
3. Mutagenicity: Bacillus subtilis DNA repair: 5mg/disc
Ecological data
No harm to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 48.56
2. Molar volume (cm3/mol): 190.9
3. Isotonic specific volume (90.2K): 438.8
4. Surface tension (dyne/cm): 27.9
5. Polarizability (10-24cm3): 19.25
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 78.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxides.
2. Found in flue-cured tobacco leaves, burley tobacco leaves, and oriental tobacco leaves.
3. Naturally found in many essential oils, such as lemongrass oil, citrus oil, neroli oil, etc.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep away from sources of fire. Store away from oxidizing agents.
Synthesis method
1. Exists in natural rose oil, lemongrass oil, coriander seed oil, citrus oil, orris root oil, neroli oil, etc. It can be separated from essential oils together with other aldehydes by generating bisulfite compounds. come out. Industrial production of decanal is obtained by distilling a mixture of decanal and formic acid barium salt under reduced pressure; or by first converting undecylenic acid into hydroxyundecylenic acid and then dry distilling it. Orthomorphic mixed fatty alcohols composed of C7-C12 can also be treated with boric acid at 210°C, and the generated water and impurities can be evaporated at the same time: the obtained decanol borate ester is decomposed with hot water, and the precipitated decanol is vacuumed Distillation and purification: Then, dehydrogenate decanol under a copper-chromium catalyst at 200-220°C and under reduced pressure to obtain decanal: industrial product decanal is a light yellow transparent liquid with an aldehyde content of not less than 70% (Shanghai Q/QBH 88-79).
2.Prepared by oxidation of decanol.
![]()
3.Prepared by reduction of decanoic acid.
![]()
4. Tobacco : BU, 56; OR, 57; FC, 18.
5. Preparation method:
![]()
In a 3L reaction bottle equipped with a stirrer, thermometer, and reflux condenser, add 94.9g (1.2mol) of pyridine and 1500mL of methylene chloride, cool to 5°C in an ice bath while stirring, and add chromium trioxide in one go. 60g (0.6mol), stir and react for 5 minutes. Slowly rise to 20°C within 1 hour. Quickly add dropwise a solution of 15.8g (0.1mol) 1-decanol (2) and 400mL methylene chloride. After the addition is complete, continue stirring the reaction for 15 minutes. The reaction solution was decanted, and the tar-like black precipitate was extracted three times with diethyl ether. The layers were combined, washed successively with 1000 mL of cold 5% sodium bicarbonate solution x 3 and water, and dried over anhydrous magnesium sulfate. The solvent is evaporated and fractionated under reduced pressure. The fractions at 96-98°C/2.1kPa are collected to obtain 9.8-10.2g of 1-decanal (1) with a yield of 63%-66%. [1]
Purpose
1. Used in organic synthesis and as spices. It is often used as a raw material for blending iris, orange blossom, jasmine, geranium, pansy, rose and other flavors. It is also used in small amounts in the manufacture of artificial bergamot oil, neroli oil and rose oil.
2.As a top fragrance, it is used in trace amounts in daily fragrances such as rose, orange blossom, jasmine, violet, lilac, and citrus. The dosage is generally less than 1%.
3. Can be used for food flavors.

 微信扫一扫打赏
微信扫一扫打赏

