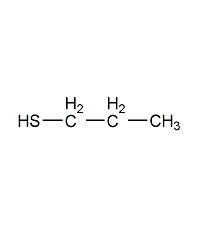
Structural formula
| Business number | 02UG |
|---|---|
| Molecular formula | C3H8S |
| Molecular weight | 76 |
| label |
1-Mercaptopropane, propylmercaptan, Propanethiol, thiopropanol, n-Propanol thio, n-propylmercaptan, propane sulfide, n-Propyl mercaptan, Propyl mercaptan, Nitrogen-containing compound solvents, For pesticide synthesis, alcohol compounds |
Numbering system
CAS number:107-03-9
MDL number:MFCD00004900
EINECS number:203-455-5
RTECS number:TZ7300000
BRN number:1696860
PubChem number:24887967
Physical property data
1. Properties: colorless or light yellow liquid with pungent odor. [1]
2. Melting point (℃): -113.3[2]
3. Boiling point (℃): 67~68[3]
4. Relative density (water=1): 0.84 (20℃)[4]
5. Relative vapor density (air=1): 2.54[5]
6. Saturated vapor pressure (kPa): 16.26 (20℃)[6 ]
7. Critical pressure (MPa): 4.6[7]
8. Octanol/water partition coefficient: 1.81[8]
9. Flash point (℃): -20.5[9]
10. Ignition temperature (℃) :287[10]
11. Solubility: Slightly soluble in water, soluble in ethanol, ether, benzene, acetone, propylene glycol, etc. [11]
Toxicological data
1. Skin/eye irritation
Standard Draize test: rabbit, eye contact: 83mg, severity of reaction: severe.
2. Acute toxicity: Rat oral LD50: 1790mg/kg; rat inhalation LC50: 7300ppm/4H; rat abdominal LD50: 515mg/kg; mouse inhalation LC50: 4010ppm/ 4H;
3. Heating decomposes to release sulfur oxide smoke, which stimulates and inhibits the central nervous system. It can stimulate the respiratory tract, cause fever, cause muscle weakness, hemolytic anemia, methemoglobinemia, hematuria, proteinuria, Cyanosis, convulsions, and loss of consciousness. The oral LD50 in mice is 3g/kg.
4. Acute toxicity [12]
LD50: 2360mg/kg (rat oral)
LC50: 22703mg/m3 (rat inhalation, 4h)
5. Irritation [13] Rabbit eye: 83mg, severe irritation.
Ecological data
1. Ecotoxicity[14] LC50: 0.06mg/L (48h) (Daphnia, static)
2 .Biodegradability No information available
3. Non-biodegradability [15] In the air, when the concentration of hydroxyl radicals is 5.00When ×105 pieces/cm3, the degradation half-life is 8h (theoretical).
Molecular structure data
1. Molar refractive index: 23.84
2. Molar volume (cm3/mol): 92.1
3. Isotonic specific volume (90.2K ): 203.3
4. Surface tension (dyne/cm): 23.7
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 9.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 7.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product decomposes when heated to release sulfur oxide smoke, which stimulates and inhibits the central nervous system. It can stimulate the respiratory tract, generate fever, and cause muscle weakness, hemolytic anemia, methemoglobinemia, hematuria, proteinuria, cyanosis, and spasm. causing loss of consciousness. Mouse LD503g/kg. The maximum allowable concentration in the air is 1.5mg/m3. The production workshop should be properly ventilated, and wear goggles, gas masks, and rubber protective clothing. This product should be kept away from fire sources and separated from oxidants and acidic substances.
2. Stability[16] Stable
3. Incompatible substances[17] Strong oxidants, acids, acid anhydrides, acid chlorides, alkali metals
4. Conditions to avoid contact[18] Heating
5. Polymerization hazard[19] No polymerization
6. Decomposition products[ 20] Hydrogen sulfide
Storage method
1. Storage precautions[21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkali metals, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Put it into a glass bottle or plastic bottle with a screw top or an iron cover, seal it tightly, and then put it into a strong wooden box. The box should be properly lined with a straw mat liner or other soft material. It is solid, and the outside of the box is reinforced with iron sheets or iron wires or iron sheets. The net weight of each barrel shall not exceed 20kg. Put it into a metal container or plastic bottle, seal it tightly, and then put it into a full-floor transparent cage wooden box or a sturdy thick cardboard box, strip, bamboo box, or fiberboard box. The inside of the box should be lined with soft materials, and the outside of the box should be tied tightly with iron sheets. The net weight of each box is 20kg, and the net weight of each bottle is 1kg.
Synthesis method
1. Obtained from the reaction of bromopropane and thiourea. Reflux the methanol solution of thiourea and bromopropane for 3 hours. After recovering the methanol, add dilute sodium hydroxide solution and reflux again for 6 hours. Then, slowly add dilute sulfuric acid dropwise, and evaporate the propanethiol at any time. When the addition of dilute sulfuric acid is completed, continue distillation until no oil droplets can be evaporated. The collected oil layer is a crude product, which is dried with anhydrous calcium chloride and then distilled. The fraction with a boiling point of 66.5-76.5°C is collected to obtain the finished product.
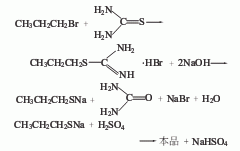
2. From halogenated propane and Derived from the action of sodium hydrosulfide.
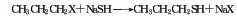
3. Propanol and hydrogen sulfide Propyl mercaptan can also be obtained through catalytic reaction at 300-380°C.
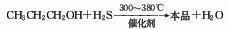
Purpose
1. It is an intermediate for synthesizing organophosphorus pesticides propothion and propionate, soil insecticides fenfos, and herbicides.
2. Used as chemical intermediates and herbicides. [22]

 微信扫一扫打赏
微信扫一扫打赏

