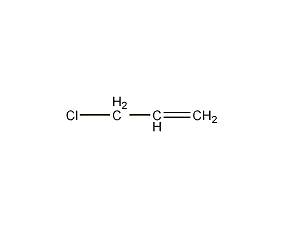
Structural formula
| Business number | 02UJ |
|---|---|
| Molecular formula | C3H5Cl |
| Molecular weight | 76.53 |
| label |
3-chloropropene, Allyl chloride, 3-Chloro-1-propene, 3-Chloro-[1]-propene, α-Chloropropylene, propenyl chloride, 3-Chloropropylene, 3-Chloro-1-propene, Allyl chloride, Aliphatic halogenated derivatives |
Numbering system
CAS number:107-05-1
MDL number:MFCD00000984
EINECS number:203-457-6
RTECS number:UC7350000
BRN number:635704
PubChem number:24845883
Physical property data
1. Properties: colorless and transparent liquid with an unpleasant pungent odor. [1]
2. Melting point (℃): -134.5[2]
3. Boiling point (℃): 44~45[3]
4. Relative density (water = 1): 0.94[4]
5. Relative vapor density (air=1): 2.64[5]
6. Saturated vapor pressure (kPa): 45.2 (20℃)[6]
7. Heat of combustion (kJ/mol): -1842.5[7]
8. Critical temperature (℃): 241[ 8]
9. Critical pressure (MPa): 4.76[9]
10. Octanol/water partition coefficient: 1.45~ 1.93[10]
11. Flash point (℃): -31.7 (CC); -28.9 (OC) [11]
12. Ignition temperature (℃): 392[12]
13. Explosion upper limit (%): 11.2[13]
14. Lower explosion limit (%): 2.9[14]
15. Solubility: insoluble in water, miscible in ethanol, ether, Most organic solvents such as chloroform and petroleum ether. [15]
16. Viscosity (mPa·s, 20ºC): 0.3374
17. Heat of evaporation (KJ/mol, b.p.): 24.86
18. Heat of formation (KJ/mol, 25ºC, gas): 29.3
19. Heat of combustion (KJ/mol, 25ºC, liquid): 1845.5
20. Specific heat capacity (KJ/(kg·K), 40ºC, liquid, calculated value): 1.65
21. Conductivity (S/m, 0ºC): <3×10-9
22. Volume expansion coefficient (K-1, 30ºC): 0.001475
23. Relative density (25℃, 4℃): 0.9311
24. Refractive index at room temperature (n25): 1.4116
25. Liquid phase standard hot melt (J·mol-1·K-1): 112.8
26. Gas phase standard entropy (J·mol-1·K-1): 310.35
27. Gas phase standard hot melt (J·mol-1·K-1): 75.06
Toxicological data
1. Irritation: Rabbit eye: 469 mg, causing irritation. Rabbit percutaneous open irritation test: 10mg/24 hours, causes irritation.
2. Acute toxicity: Rat oral LD50 is 700mg/kg; rat inhalation LD5O: 11000mg/m3, 2 hours; rabbit transdermal LD5O: 2066 mg/kg;
3. It is of low toxicity. Can strongly stimulate the skin,The reaction heat is used to preheat propylene. The single-pass conversion rate of propylene is 25%. The conversion of chlorine is into stoichiometric amount. The total yield of allyl chloride is 80-85%. In addition to the main product allyl, there are also 1,2-dichloropropene, 1, 3-Dichloropropene, hydrogen chloride, 1,2,3-trichloropropane and other small amounts of by-products. The chlorination reactant is quenched to 50-100°C to remove hydrogen chloride and propylene, and then is fractionated to obtain propylene chloride. For an allyl chloride device with an annual output of 13,500 tons, each ton of product consumes approximately 700kg of propylene and 1,120kg of chlorine. 2. Oxychlorination method Using propylene as raw material and tellurium as catalyst, allyl chloride is obtained through the following reaction; propylene, hydrochloric acid and oxygen are mixed in the ratio of 2.5-1:1:1-0.2 (molar ratio). The reaction was carried out at 240°C and 0.101MPa. The reactor is a fluidized bed, the catalyst is Te V2 O5 H3 PO4 carried on the carrier, and nitrogen-containing substances are added as promoters. The selectivity is over 90%, and the space-time yield of the fluidized bed is greater than 100g allyl chloride/L catalyst·hour. In small batch production, it can be obtained by chlorination of allyl alcohol; add sulfuric acid to allyl alcohol, cuprous chloride and hydrochloric acid at 10-20°C. After the addition is completed, keep warm for 5 hours. Leave to stand for layering, remove the mixed acid from the lower layer, wash the upper layer once with water, once with 5% sodium carbonate solution, and once with water. After all the water is separated, collect the fractions above 40°C by distillation to obtain allyl chloride. The yield is 73%.
2. High-temperature chlorination method: It is produced by high-temperature oxidation of propylene. Reaction equation:
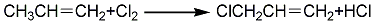
Put dry propylene After preheating at 350~400℃, liquid chlorine is heated and vaporized, and the two materials are mixed and reacted under high-speed injection. The ratio of propylene to chlorine is 4~5:1 (molar ratio), and the reactor residence time is 1.5 ~2s, reaction temperature 470~500℃. The reaction product is quenched to 50-100°C to remove HCl and propylene, and then the product is obtained by fractionation. This method is adopted by most domestic and foreign production companies.
3. Propylene oxychlorination method: Propylene, hydrogen chloride and oxygen are mixed in a ratio of 2.5 ~ (1:1:1) ~ 0.2 (molar ratio) in a fluidized bed reactor, and a catalyst is selected It is Te, V2O5 or H3PO4 loaded on the carrier, and nitrogen-containing substances are added as accelerator, and the atmospheric pressure oxygen chlorination reaction is carried out at 240 to 260°C to prepare 3-chloropropene. Reaction equation:
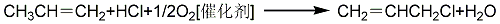
4. Allyl Alcohol chlorination reaction equation:

Drop sulfuric acid into allyl alcohol, cuprous chloride and hydrochloric acid at 10-20°C. After the dropwise addition is completed, keep the temperature for 5 hours and let it stand to separate layers. Wash the upper layer once with water, 5% sodium carbonate solution and water. After the water is separated, distillation collects the fraction above 40°C, which is 3-chloropropene. This method is suitable for small batch production.
Purpose
1. This product is an organic synthetic raw material. It is used in pesticides to synthesize N, N-dimethylpropylene amine, the intermediate for insecticides, insecticides, and feniticide, and propylene, the intermediate for pyrethroids. Alcohols and ketones, as well as solvents for special reactions.
2. This product has the reactivity of both olefins and halogenated hydrocarbons, and is an organic synthesis intermediate for glycerol, epichlorohydrin, propylene alcohol, etc. It is also used as a raw material for pesticides and medicines. It can also be used as raw material for synthetic resins, coatings, binders, plasticizers, stabilizers, surfactants, lubricants, soil conditioners, spices and other fine chemicals. It is mainly used to make epichlorohydrin, glycerin, Chloropropanol, propylene alcohol, pesticides and insecticides, medicines, resins, coatings, adhesives, sodium propylene sulfonate, lubricants, etc.
3. Used in organic synthesis and pharmaceutical industry. Also used in the preparation of intermediates such as plastics.
4. Used as intermediates for pharmaceuticals, pesticides, plastics, etc. [32]

 微信扫一扫打赏
微信扫一扫打赏

