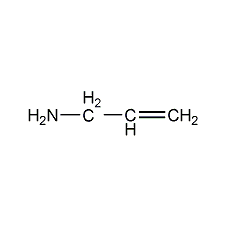
Structural formula
| Business number | 02UP |
|---|---|
| Molecular formula | C3H7N |
| Molecular weight | 57.09 |
| label |
2-propen-1-amine, 3-amino-1-propene, 2-Propen-1-ylamine, 3-Amin-opropylene, 3-Aminopropylene, polymer modifiers, Nitrogen-containing compound solvent |
Numbering system
CAS number:107-11-9
MDL number:MFCD00008199
EINECS number:203-463-9
RTECS number:BA5425000
BRN number:635703
PubChem number:24847185
Physical property data
1. Properties: colorless liquid with strong ammonia and burning smell. [1]
2. Melting point (℃): -88.2[2]
3. Boiling point (℃): 55~58[3]
4. Relative density (water = 1): 0.76[4]
5. Relative vapor density (air=1): 2.0[5]
6. Saturated vapor pressure (kPa): 25.7 (20℃)[6]
7. Heat of combustion (kJ/mol): -2207.5[7]
8. Critical pressure (MPa): 5.17[ 8]
9. Octanol/water partition coefficient: 0.03[9]
10. Flash point (℃): -29 (CC)[10]
11. Ignition temperature (℃): 371[11]
12. Explosion Upper limit (%): 22.0[12]
13. Lower limit of explosion (%): 2.2[13]
14 .Solubility: Soluble in water, ethanol, ether, and chloroform. [14]
Toxicological data
1. Acute toxicity[15]
LD50: 102mg/kg (rat oral); 35mg/kg (rabbit dermal )
LC50: 177ppm (rat inhalation, 8h)
2. Irritation [16]
Rabbit transdermal: 500mg (24h), severe stimulation.
Rabbit eye: 50mg (20sec), severe irritation.
3. Subacute and chronic toxicity [17] Subacute and chronic toxicity manifests as myocarditis, liver and kidney congestion, and lung lesions.
4. Mutagenicity [18] Cytogenetic analysis: Rat oral administration 2500ng/kg
Ecological data
1. Ecotoxicity[19] EC50: 16mg/L (5, 15min) (photobacteria, Microtox test)
2. Biodegradability[20] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, 1%~81%.
3. Non-biodegradability[21] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 6.8h (theoretical).
Molecular structure data
1. Molar refractive index: 19.20
2. Molar volume (cm3/mol): 76.8
3. Isotonic specific volume (90.2K ): 169.5
4. Surface tension�� (dyne/cm): 23.7
5. Dielectric constant:
6. Dipole moment (10-24cm3 ):
7. Polarizability: 7.61
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 17.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: It has the chemical properties of primary amines and can also undergo various reactions on double bonds. For example, it adds with halogen and hydrogen halide to form halopropylamine. Reacts with benzene to obtain β-aminocumene. Allylamine does not polymerize under the action of free radical catalysts. Reacts with cyclopentadiene to obtain 2,5-methylene-1,2,5,6-tetrahydrobenzylamine.
2. Stability[22] Stable
3. Incompatible substances[23] Acids, acid chlorides, acid anhydrides, strong oxidants, carbon dioxide
4. Conditions to avoid contact[24] Heating
5. Polymerization hazard[25] Polymerization
6. Decomposition products[26] Ammonia
Storage method
Storage Precautions[27] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29°C. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Allylamine can be obtained by reacting allyl chloride with ammonia: The reaction is carried out in equipment with a reflux device, and the reflux reaction of allyl thioisocyanate and 20% hydrochloric acid is carried out for 15 hours. The reaction was concentrated, diluted with water when crystals appeared, and then the allylamine was distilled off while adding alkali solution dropwise. The collected crude product can be refined by fractionation.
Purpose
1. Used as polymer modifier and diuretic, raw material for organic synthesis, etc.
2. Intermediates used in the manufacture of pharmaceuticals, and solvents for organic synthesis and production. [28]

 微信扫一扫打赏
微信扫一扫打赏

