Structural formula
| Business number | 02UY |
|---|---|
| Molecular formula | C2H6O2 |
| Molecular weight | 62.07 |
| label |
glycol, Ethylene Glycol, 1,2-ethanediol, Glycol, Ethylene alcohol, Aliphatic alcohols, ethers and their derivatives, Raw materials and intermediates used in ink |
Numbering system
CAS number:107-21-1
MDL number:MFCD00283324
EINECS number:203-473-3
RTECS number:KW2975000
BRN number:505945
PubChem number:24859450
Physical property data
1. Properties: Colorless, transparent and slightly viscous liquid. The taste is slightly sweet. Easy to absorb moisture. Odorless.
2. Boiling point (ºC, 101.3kPa): 197.3
3. Melting point (ºC): -13~-11
4. Relative density (g /mL, 0/4ºC): 1.1274
5. Relative density (g/mL, 10/4ºC): 1.1204
6. Relative density (g/mL, 20/4ºC ): 1.1135
7. Relative vapor density (g/mL, air=1): 2.14
8. Refractive index (15ºC): 1.43312
9 .Refractive index (20ºC): 1.4318
10. Viscosity (mPa·s, -7ºC): 86.9
11. Viscosity (mPa·s, 16ºC): 25.66
12. Viscosity (mPa·s, 38ºC): 10.38
13. Flash point (ºC): 111.1
14. Fire point (ºC): 118
15. Heat of evaporation (KJ/mol): 57.11
16. Heat of fusion (KJ/kg): 187.2
17. Heat of generation (KJ/mol): -452.6
18. Heat of combustion (KJ/mol, 20ºC, constant pressure): 1186.1
19. Heat of combustion (KJ/kg, 20ºC, constant pressure): 1186.1
p>
20. Specific heat capacity (KJ/(kg·K), constant pressure): 2.35
21. Electrical conductivity (S/m, 25ºC): 1.07×10-6
22. Thermal conductivity (W/(m·K), 20ºC): 0.28888
23. Lower explosion limit (%, V/V): 3.2
24. Explosion upper limit (%, V/V): 15.3
25. Volume expansion coefficient (K-1): 0.000566
26. Freezing point (ºC): -11.5
27. Autoignition point (ºC): 412.8
28. Vapor pressure (kPa, 50ºC): 0.09
29. Solubility: miscible with water, ethanol, acetone, acetic acid, glycerol, pyridine, etc. But it is insoluble in chloroform, ether, benzene, carbon disulfide, etc., and insoluble in hydrocarbons, chlorinated hydrocarbons, oils, rubber, natural resins, etc. Can dissolve salt, zinc chloride, potassium carbonate, potassium chloride, potassium iodide, potassium hydroxide and other inorganic compounds.
30. Relative density (25℃, 4℃): 1.1100
31. Refractive index at room temperature (n25): 1.4306
32. Critical temperature (ºC): 446.85
33. Critical pressure (MPa): 8
34. Solubility parameter (J·cm-3)0.5: 34.478
35. van der Waals area (cm2·mol-1): 5.620 ×109
36. van der Waals volume (cm3·mol-1): 36.540
37. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -1256.96
38. Gas phase standard claimed heat (enthalpy) (kJ·mol -1): -387.56
39. Gas phase standard entropy (J·mol-1·K<suthings. Diethylene glycol can be used as a humidifier, plasticizer, sizing agent, printing ink solvent, natural gas dehydration desiccant and aromatic hydrocarbon extraction solvent. Diethylene glycol dinitrate is similar to ethylene glycol dinitrate and is also an important industrial explosive. High molecular weight polyethylene glycols, which range from colorless and transparent viscous liquids to waxy solids depending on their molecular weight, are also a useful class of derivatives. It is used as lubricant, moisture retaining agent, solvent and intermediate in the rubber and food industries. It is also used in the preparation of cosmetics and auxiliaries in textile, paper and other fields. There are many types of esters of ethylene glycol, which are widely used as solvents. Glycol esters of long-chain fatty acids have surface-improving properties and can be used alone or in combination with surfactants as emulsifiers, stabilizers, dispersants, moisturizers, foaming agents, and suspending agents. Ethylene glycol reacts with urea to form cycloethylene urea, which is used in the textile industry. Disodium ethylene glycol reacts with 1,2-dibromoethane to form dioxane, a special solvent. Use different oxidizing agents or reaction conditions for ethylene glycol. After oxidation, glycolaldehyde, glyoxal, glycolic acid, oxalic acid, etc. can be obtained.
2. Used as analytical reagent, liquid chromatography eluent, and electrochemical analysis of non-aqueous media. Also used as solvent, antifreeze, and in organic synthesis.
3. Used in the electronics industry. Can be used as a cleaning and degreasing agent.
4. Ethylene glycol is a multi-functional reagent commonly used in chemical laboratories. It can form acetals or ketals with aldehydes and ketones to protect carbonyl groups; it can be used as a solvent for Diels-Alder reaction or Fischer indole synthesis; it can be used to prepare tertiary alcohols from trialkyl boron, etc.
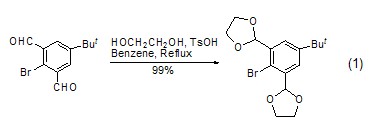
Formation of acetals and ketals Ethylene glycol is one of the most commonly used compounds to protect carbonyl groups. Acetal and ketal groups are stable to bases, Grignard reagents, alkyllithium reagents, metal hydrides, Wittig reagents, and reaction conditions such as oxidation, reduction, bromination, and esterification. The order of difficulty in forming acetal or ketal is: aldehyde > acyclic aliphatic ketone > six-membered cyclic ketone > five-membered cyclic ketone > α,β-unsaturated ketone > α-Mono- or disubstituted ketone >> Aromatic ketone. Ketone reactions require stronger acidic conditions than aldehydes, such as HCl and TsOH (Formula 1)[1~4].
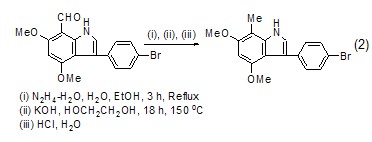
Used as reaction solvent Ethylene glycol is a good solvent for many reactions. It has a high boiling point (197.6 oC) and a low melting point (–13 oC), making the operating temperature The range is greatly expanded, and it can be used as a solvent for reactions above 150 oC (Formula 2) [5,6].
Ethylene glycol can also be synthesized as Solvent for condensed ring compounds, for example, under the catalysis of Ru catalyst, an alkyne derivative reacts with a substituted aniline to synthesize an indole ring[7]. Compared with traditional methods, the reaction conditions of this method are milder (Equation 3).
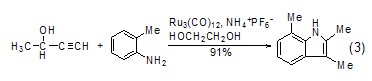
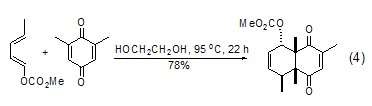
When diene and dienophile undergo Diels-Alder reaction, ethylene glycol is more superior as a solvent than benzene, MeCN, DMSO, MeOH, etc., and the reaction rate is higher (Formula 4)[8, 9].
Reaction to form ester As a compound with an alcoholic hydroxyl group, ethylene glycol can react with carboxylic acid compounds to form ester (Formula 5)[10].

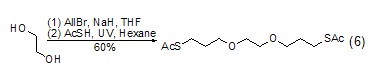
React to form ether Ethylene glycol can also react with alcohols, halides, etc. to form ethers (Formula 6)[11,12].

 微信扫一扫打赏
微信扫一扫打赏

