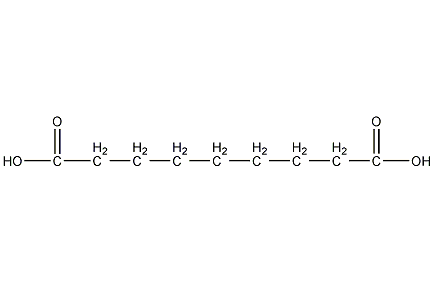
Structural formula
| Business number | 03HK |
|---|---|
| Molecular formula | C9H16O4 |
| Molecular weight | 188.22 |
| label |
1,7-Azelaic acid, Heptane-1,7-dihydroxy acid, Azalea acid, Nonanedioic acid, 1,7-Heptanedicarboxylic acid, Anchoic acid, aliphatic compounds, acidic solvent |
Numbering system
CAS number:123-99-9
MDL number:MFCD00004432
EINECS number:204-669-1
RTECS number:CM1980000
BRN number:1101094
PubChem ID:None
Physical property data
1. Properties: Appearance: white to yellowish monoclinic prisms, needle crystals or powder.
2. Density (g/mL, 25/4℃): (d110.6/4) 1.225
3. Relative vapor density (g/mL, air=1) : Undetermined
4. Melting point (ºC): 106.5
5. Boiling point (ºC, normal pressure): 357.1, 225ºC (13.33kPa)
6 . Refractive index at room temperature (n25): 1.4303100
7. Refractive index (n111D): 1.4303
8. Flash point (ºC): 210
9. Crystal phase standard combustion heat (enthalpy) (kJ ·mol-1): -4773.94
12. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -1054.28
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion ( KJ/mol): Undetermined
14. Critical temperature (ºC): 570.85
15. Critical pressure (MPa): 2.72
16. Oil and water ( Log value of the partition coefficient (octanol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/ V): Undetermined
19. Solubility: Easily soluble in hot water, alcohol and hot benzene, slightly soluble in water, ether and benzene.
Toxicological data
1. Skin/eye irritation: Rabbit skin standard Drez eye dye test: 500mg/24H has a slight irritating effect on the skin.
Standard Drez eye dye test on rabbit eyes: 3mg has a slight irritating effect on the eyes
2. Acute toxicity: rat oral LD50: >5gm/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 46.87
2. Molar volume (cm3/mol): 166.3
3. Isotonic specific volume (90.2K ): 433.8
4.Surface tension (dyne/cm): 46.2
5. Polarizability (10-24cm3): 18.58
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.6
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 8
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 74.6
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 147
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Decomposed by high heat and emit irritating smoke. Powder and air can form an explosive mixture. When it reaches a certain concentration, it will explode when encountering Mars.
2. Found in flue-cured tobacco leaves, burley tobacco leaves, and oriental tobacco leaves.
Storage method
Packed in sacks or woven bags lined with plastic bags. Store in a cool and ventilated place.
Synthesis method
1. Brief description of production method: Obtained from oleic acid oxidized by nitric acid or ozone. It can also be obtained by cracking industrial castor as raw material. Azelaic acid can be produced by condensing 1,5-dibromopentane with acetonitrile, or by using glutarate monomethyl cyanide as raw material via ketone azelaic acid dimethyl ester.
2. Tobacco: OR, 44; BU, 9; OR, 41; BU, 26.
3. Preparation method:
In a reaction bottle equipped with a stirrer and a reflux condenser, add 100g of potassium hydroxide, 1000mL of 95% ethanol, and 500g of castor oil, and stir Reflux for 3 hours, pour the reaction solution into 3L of water, and acidify with dilute acid composed of 100mL of concentrated sulfuric acid and 300mL of water. The oil layer was separated, washed twice with hot water, and dried over anhydrous magnesium sulfate. The desiccant was filtered off to obtain about 480 g of crude 12-hydroxyoctadecene-9-acid (2). This product polymerizes in solution and should not be left for long periods of time. It is best to use it in time for the next reaction. In a reaction bottle equipped with a stirrer, thermometer, and reflux condenser, add 625g (3.5mol) of potassium permanganate and 7.5L of water, and stir vigorously at 35°C to dissolve. Add a solution consisting of 240g (0.8mol) of 12-hydroxyoctadecene-9-acid (2) dissolved in 1.6L of water and 64g of potassium hydroxide at one time. The reaction is exothermic and the temperature rapidly rises to about 75°C. Continue stirring the reaction for 0.5h. A large amount of foam will be generated during the reaction, and a defoaming agent can be added if necessary. Excess MnO2 is removed while hot, and the filter cake is washed twice with hot water. The filtrate is acidified with dilute sulfuric acid. Concentrate under reduced pressure to about 2L, cool and crystallize in the refrigerator. Filter the precipitated solid, wash with cold water, and dry to obtain 35-38g of azelaic acid (1). Recrystallize it with 15 times hot water, decolorize it with activated carbon, filter out the crystals after cooling, and dry to obtain pure azelaic acid (1). 1) 23~27g, mp104~106℃, yield 31%~35%. [1]
Purpose
1. It is mainly used to produce dioctyl azelate plasticizer, and can also be used as raw material for the production of spices, lubricants, oils, and polyamide resins.
2.Azalea acid has antibacterial properties and can be used as a food preservative. Use in mouthwash is beneficial to the prevention and treatment of dental caries, and use in soap can avoid cracking on the surface of the soap body. It has good permeability to the skin and can be used in cream cosmetics to increase the absorption function of the skin. It has various medicinal properties and is used in dermatological plasters. It has skin lightening and whitening functions. Rhododendronic acid or its zinc salts are used in hair care products in combination with vitamin B6. It is suitable for the treatment of male hormonal alopecia with strong endocrine and stimulates hair growth at the same time.

 微信扫一扫打赏
微信扫一扫打赏

