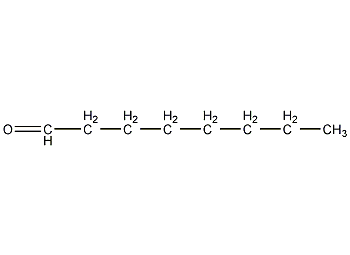
Structural formula
| Business number | 03HV |
|---|---|
| Molecular formula | C8H16O |
| Molecular weight | 128.21 |
| label |
1-octanal, n-Octyl aldehyde, suet, Aldehyde C8, Caprylaldehyde, n-Caprylaldenyde, n-Octaldenhyde, food additives, Flavor enhancer |
Numbering system
CAS number:124-13-0
MDL number:MFCD00007029
EINECS number:204-683-8
RTECS number:GR7780000
BRN number:1744086
PubChem number:24898024
Physical property data
1. Properties: colorless liquid with strong fruity aroma. [1]
2. Melting point (℃): -23[2]
3. Boiling point (℃): 163.4[3]
4. Relative density (water = 1): 0.82[4]
5. Relative vapor Density (air=1): 4.41[5]
6. Saturated vapor pressure (kPa): 0.266 (20℃)[6]
7. Critical pressure (MPa): 2.96[7]
8. Octanol/water partition coefficient: 2.65[8]
9. Flash point (℃): 51 (CC) [9]
10. Ignition temperature (℃): 196[10]
11. Solubility: Insoluble in glycerol, soluble in ethanol, ether, fixed oil, and propylene glycol. [11]
Toxicological data
1. Acute toxicity[12] LD50: 5630mg/kg (rat oral); 6350mg/kg (rabbit transdermal)
2. Irritation[13]
Rabbit transdermal: 500mg (24h), mild irritation.
Rabbit eye: 100mg, mild irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 39.29
2. Molar volume (cm3/mol): 157.9
3. Isotonic specific volume (90.2K ): 359.3
4. Surface tension (dyne/cm): 26.8
5. Polarizability (10-24cm3): 15.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 59.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain chemical bond configurationNumber of centers: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[14] Stable
2. Incompatible substances[15] Strong oxidizing agent, strong reducing agent, strong alkali
3. Polymerization hazard[16] No polymerization
Storage method
Storage Precautions[17] Stored in a cool, ventilated warehouse. The storage temperature should not exceed 37°C. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Octanol or octanoic acid can be used as raw materials. Octanol is oxidized in the presence of copper-chromium catalyst to generate octanal. There are various methods of producing octanoic acid using octanoic acid, such as mixing octanoic acid vapor with excess formic acid vapor and using titanium oxide or manganese oxide catalyst at 300°C to produce octanal with a yield of 90%.
2. Using n-octanol as raw material, it is produced through catalytic oxidation reaction.
![]()
3. Using n-octanoic acid as raw material , prepared by reduction reaction.
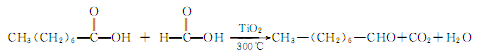
4. Preparation method:
p>
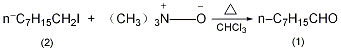
Equipped with stirrer and reflux condenser (Calcium chloride drying tube) and dropping funnel, add 30g (0.4mol) anhydrous trimethylamine oxide and 100mL dry chloroform. Slowly add 48g (0.2mol) of n-octyl iodide (2) dropwise while stirring. When starting to add, heat to 40~50°C to initiate the reaction. After the reaction starts, the reaction will exotherm. Control the dropping speed and maintain the temperature of the reaction solution at 50°C. ℃. It takes about 20 to 30 minutes to finish adding. After adding, reflux for 20 minutes. After cooling, slowly add 110 mL of 2 mol/L sulfuric acid, separate the chloroform layer, wash with water, wash with 2 mol/L sodium carbonate, and then wash with water. Dry over anhydrous sodium sulfate. Concentrate under reduced pressure and distill the residue. The yield is 155-165°C fraction, and 12-12.5g of crude product is obtained. Re-fractionate under reduced pressure and collect the fraction at 69~71°C/2.53kPa to obtain 10.6~11g of colorless oily liquid (1) with a yield of 41.5%~43%. [19]
5. Preparation method:
![]()
Refer to the above synthesis method of hexanal (reference book 316 Page), use 25g of octanitrile (2) (bp87℃/1.67kpa), 57g of anhydrous stannous chloride, 200mL of anhydrous ether, steam distillation to evaporate octanal, and finally distill under reduced pressure to collect 65℃/ From the 1.8kPa fraction, octanal (1) was quantitatively obtained. [20]
6. Preparation method:
Add 180mL of boron with a concentration of 1.0mol/L into the reaction bottle Sodium hydride-diglyme solution, 2-methyl-2-butene 33.6 (0.48 mol), cooled in ice bath. Slowly add boron trifluoride-diethyl ether solution (0.24 mol) dropwise. After the addition is completed, react at 0°C for 2 hours, and then put it into an ice-salt bath. At this time, the hydroboration reagent Sia2BH is generated. Enter the solution of 22.0g (0.2mol) of octyne-1(2) dissolved in 20mL of diethylene glycol dimethyl ether as quickly as possible, and be careful to keep the temperature of the reaction solution no higher than 10°C. Slowly rise to room temperature to complete the hydroboration reaction. Maintain 0°C and slowly add 150 mL of 15% hydrogen peroxide for oxidation. Pay attention to maintaining the pH of the reaction solution at 7 to 8 by adding 3 mol/L sodium hydroxide solution. The reaction solution was adjusted to neutrality and steam distilled. The effluent was extracted with diethyl ether, dried over anhydrous magnesium sulfate, and distilled. The fractions at 83-85°C/4.39kPa were collected to obtain 18 g of compound (1) with a yield of 70%. [21]
Purpose
1. Octanal has a strong fruity aroma and a pleasant sweet orange aroma when extremely dilute. my country’s GB 2760-86 stipulates that it is a temporarily allowed edible spice and is mainly used for the blending of citrus spices. , the dosage in beverages, ice cream, candy, bread, etc. is 1.4-4.4ppm.
2. Can be used as perfume for soaps and detergents. Octanal is also an intermediate in organic synthesis.
3. Used in the manufacture of surfactants such as soap, soap, stearic acid, stearates, glycerin, fatty amines, fatty alcohols, fatty acid sulfonates, etc., as well as softeners, greases, etc. .
4. As a top fragrance, it can be used in small amounts in daily flavors such as carnation, rose, orange blossom, and orange cologne. It can also be used in small amounts in food flavors such as cream, chocolate, apricots, oranges, and cheese. middle.
5. Used for making flavors, fragrances and organic synthesis. [18]

 微信扫一扫打赏
微信扫一扫打赏

