
Structural formula
| Business number | 02WQ |
|---|---|
| Molecular formula | C4H6O3 |
| Molecular weight | 102.09 |
| label |
Acetic anhydride, acetic anhydride, acetic anhydride, acetic anhydride, anhydrous acetic acid, Acetic anhydride, Anhydrous acetic acid, Acetyl oxide, Acetic acid anhydride, Ethanoic anhydride, acetylating agent, dehydrating agent, Acid anhydride solvent |
Numbering system
CAS number:108-24-7
MDL number:MFCD00008705
EINECS number:203-564-8
RTECS number:AK1925000
BRN number:385737
PubChem number:24854587
Physical property data
1. Characteristics: colorless and transparent liquid with a pungent odor, and its vapor is a tear gas. [1]
2. Melting point (℃): -73.1[2]
3. Boiling point (℃): 139~140[3]
4. Relative density (water=1): 1.08[4]
5. Relative vapor density (air=1): 3.52[5]
6. Saturated vapor pressure (kPa): 1.33 (36℃)[6]
7. Heat of combustion (kJ/mol): -1804.5[7]
8. Critical temperature (℃): 326[ 8]
9. Critical pressure (MPa): 4.36[9]
10. Octanol/water partition coefficient: -0.58 [10]
11. Flash point (℃): 49 (CC) [11]
12. Ignition Temperature (℃): 316[12]
13. Explosion limit (%): 10.3[13]
14 .Lower explosion limit (%): 2.7[14]
15. Solubility: soluble in cold water, soluble in ethanol, ether, and benzene. [15]
16. Refractive index (15ºC): 1.39299
17. Refractive index (n20ºC): 1.3903
18 . Viscosity (mPa·s, 15ºC): 0.971
19. Viscosity (mPa·s, 30ºC): 0.783
20. Flash point (ºC, opening): 64.4
21. Flash point (ºC, closed l): 49.4
22. Fire point (ºC): 392
23. Heat of vaporization (KJ/mol, b.p.) : 38.23
24. Heat of formation (KJ/mol, 25ºC, liquid): -624.50
25. Heat of combustion (KJ/mol, 25ºC, liquid): -1786.9
26. Specific heat capacity (KJ/(kg·K), 30ºC, constant pressure): 1.88
27. Thermal conductivity (W/(m·K), 15ºC): 0.2985
28. Thermal conductivity (W/(m·K), 30ºC): 0.2921
29. Thermal conductivity (W/(m·K), 60ºC): 0.2788
30. Body expansion coefficient (K-1, 20ºC): 1.120×10-3
31. Body expansion coefficient (K-1,55ºC): 1.160×10-3
32. Eccentricity factor: 0.840
33. Gas phase Standard heat of combustion (enthalpy) (kJ·mol-1): -1859.3
34. Gas phase standard claims heat (enthalpy) (kJ·mol-1 sup>): -572.5
35. Gas phaseCrotonaldehyde is condensed into polyester, which is then hydrolyzed and refined to obtain the finished product.

This method Using triethyl phosphate as a catalyst, gas phase catalytic cracking generates ketene at 600~800℃ and 13.3~21.3kPa (absolute pressure). The heat absorption is 146kJ/mol. The single-pass conversion rate of acetic acid is 70%~80%. Ethylene The selectivity for ketones is 90% to 95%.
2. Malonic acid method: obtained by condensation and decarboxylation of malonic acid and crotonaldehyde.

3. The acetone method consists of acetone and Crotonaldehyde is condensed and then dehydrogenated.
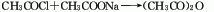
4. Butadiene method Butadiene and acetic acid are used as raw materials. In the presence of manganese acetate catalyst, they are compressed and combined at 140°C to prepare γethylene γbutyrolactone. Butyrolactone is ring-opened under the action of acidic ion exchange resin to obtain sorbic acid.
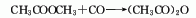
5. Acetic acid cracks at high temperatures Ketone can be obtained, and then absorbed with acetic acid to obtain acetic anhydride:

High-purity acetic anhydride can be obtained by adding benzene to remove acetic acid through azeotropic distillation or by efficient vacuum distillation during refining.
Purpose
1. Acetic anhydride is an important acetylating agent and is also used in the manufacture of medicines, spices, dyes, plasticizers, etc. Used as acetylating agent and dehydrating agent in organic synthesis, it can form acetate and acetamide compounds from alcohol, phenol, ammonia and amine respectively. In the presence of Lewis acid, acetic anhydride can also acetylate aromatic hydrocarbons or alkenes. In the presence of sodium acetate, acetic anhydride and benzaldehyde undergo a condensation reaction to form cinnamic acid. Acetic anhydride is used to make cellulose acetate, acetate plastics, and non-flammable film films; in the pharmaceutical industry, it is used to make synmycin, furazole, dibazole, caffeine, aspirin, and sulfonamide drugs; In the dye industry, it is mainly used to produce dispersed dark blue HCL, dispersed red S-SWEL, dispersed yellow brown S-2REL, etc.; in the spice industry, it is used to produce coumarin, bornyl acetate, sunflower musk, cypress acetate, Rosin acetate, phenethyl acetate, geranyl acetate, etc.; acetyl peroxide produced from acetic anhydride is an initiator and bleaching agent for polymerization reactions.
2. Test alcohols, aromatic primary amines and secondary amines. Used in organic synthesis, dyes, pharmaceutical industry and manufacturing acetyl compounds.
3. Used as a solvent and dehydrating agent, and also an important acetylation reagent and polymer initiator. Application The final product is cellulose acetate and cellulose acetate plastic. This fiber is mostly used in the manufacture of cigarette filters, fabrics in the shipbuilding industry and daily fabrics. It can also be used to manufacture the cyclone explosive triptylene trinitramine. It can also be used in medicine, dyes, spices, etc.
4. Used as acetylating agent, and used in the manufacture of drugs, dyes, and acetate fiber. [24]

 微信扫一扫打赏
微信扫一扫打赏

