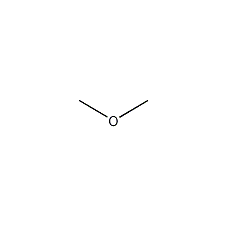
Structural formula
| Business number | 037K |
|---|---|
| Molecular formula | C2H6O |
| Molecular weight | 46.07 |
| label |
oxodimethane, Methyl ether, oxodimethane, methoxymethane, Dimethyl ether, Methoxymethane, refrigerant, foaming agent, Ether solvents, leaching agent, Extracting agent, alkylating agent |
Numbering system
CAS number:115-10-6
MDL number:MFCD00008494
EINECS number:204-065-8
RTECS number:PM4780000
BRN number:1730743
PubChem ID:None
Physical property data
1. Characteristics: colorless gas with a unique ether smell. [1]
2. Melting point (℃): -141.5[2]
3. Boiling point (℃): -24.8[3]
4. Relative density (water = 1): 0.61[4]
5. Relative Vapor density (air=1): 1.6[5]
6. Saturated vapor pressure (kPa): 533.2 (20℃)[6]
7. Heat of combustion (kJ/mol): -1453[7]
8. Critical temperature (℃): 127[8 ]
9. Critical pressure (MPa): 5.33[9]
10. Octanol/water partition coefficient: 0.10[10]
11. Flash point (℃): -41 (CC) [11]
12. Ignition temperature (℃): 350[12]
13. Explosion upper limit (%): 27[13]
14. Lower explosion limit (%): 3.4[14]
15. Solubility: soluble in water, ethanol, and ether. [15]
16. Refractive index (20ºC): 1.3018s
17. Gas viscosity (mPa·s, 20ºC): 85.1
18. Heat of evaporation (KJ/mol,-24.8ºC): 467.4
19. Heat of fusion (KJ/kg): 107.3
20. Heat of formation (KJ/mol, gaseous): -185.5
21. Critical density (g·cm-3): 0.275
22 .Critical volume (cm3·mol-1): 168
23. Critical compression factor: 0.270
24. Eccentricity factor: 0.204
25. Solubility parameter (J·cm-3)0.5: 17.572
26.van van der Waals area (cm2·mol-1): 4.840×1010
27. van der Waals volume (cm3·mol-1): 31.040
28. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -1460.5
29. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -184.1
30. Gas phase Standard entropy (J·mol-1·K-1): 267.34
31. Gas phase standard free energy of formation (kJ·mol -1): -112.9
32. Gas phase standard hot melt (J·mol-1·K-1): 65.57
Toxicological data
1. AcuteSafety: Rat inhalation LD5O: 308gm/m3;
2. Other multiple dose toxicity: Rat inhalation TDLO: 2pph/6H/30W-I;
3. Inhalation toxicity It has a depressant effect on the central nervous system. Inhalation can cause anesthesia and suffocation, and the anesthetic effect is weaker than ether. Irritating to skin. Mixing with air can form explosive mixtures, and the allowable concentration in air is 400mg/kg.
4. Acute toxicity[16]
LC50: 308000mg/m 3(Rat inhalation)
5. Irritation No data available
6. Subacute and chronic toxicity[17] Rats were inhaled 2% methyl ether for 6 hours a day, 5 days a week for 30 weeks. Weight gain, blood, urine and histopathological examination were all No obvious abnormalities were found, but serum alanine, aspartate, and aspartate aminotransferase were increased, indicating hepatotoxicity.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradable[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 5.4d (theoretical).
Molecular structure data
1. Molar refractive index: 13.15
2. Molar volume (cm3/mol): 67.9
3. Isotonic specific volume (90.2K ): 131.3
4. Surface tension (dyne/cm): 14.0
5. Polarizability (10-24cm3):5.17
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 2.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Methyl ether has methylation reaction properties. Reacts with carbon monoxide to generate acetic acid or methyl acetate; reacts with carbon dioxide to generate methoxyacetic acid; reacts with hydrogen cyanide to generate acetonitrile. Can be chlorinated into various chlorinated derivatives. Can form explosive mixtures with air.
2. Stability[19] Stable
3. Incompatible substances[20] Strong oxidants, strong acids, halogens, sulfur, sulfides
4. Conditions to avoid contact [21] Contact Air, light
5. Aggregation hazard[22] No aggregation
Storage method
Storage Precautions[23] Store in a cool, ventilated warehouse dedicated to flammable gases. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants, acids, and halogens, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with leakage emergency response equipment.
Synthesis method
1. It is mainly obtained as a by-product in the production of synthetic methanol. However, with the widespread application of low-pressure methanol technology based on copper-based catalysts, the content of dimethyl ether in the crude methanol product is very small. In small-scale production, methanol catalytic dehydration method can be used, including liquid phase method and gas phase method. The liquid phase method is to heat a mixture of methanol and sulfuric acid to obtain dimethyl ether. The gas phase method is to pass methanol vapor through a solid catalyst of alumina or crystalline aluminum silicate (ZSM-5 molecular sieve can also be used), and dehydrate the gas phase to generate dimethyl ether. In the laboratory, it can be obtained from the decomposition of trimethyl orthoformate using ferric chloride as a catalyst (yield 95%). High-purity methyl ether can be produced by Williamson synthesis from methyl iodide and sodium methoxide.
Purpose
1. Mainly used as a methylating agent to produce dimethyl sulfate, and can also be used to synthesize N,N-dimethylaniline, methyl acetate, acetic anhydride, ethylene dimethyl ester and ethylene, etc.; it can also be used Used as a substitute for alkylating agents, refrigerants, foaming agents, solvents, leaching agents, extraction agents, anesthetics, fuels, civil compound ethanol and Freon aerosols. Used in hair care, skin care, pharmaceuticals and coatings as various aerosol propellants. Fuel additives promoted abroad have many unique uses in the pharmaceutical, dye, and pesticide industries.
2. It is widely used in pharmaceutical, dye, pesticide and other industries to produce dimethyl sulfate, methyl acetate, ethylene, etc. It can also be used as aerosol propellant or refrigerant. High concentrations of methyl ether can also be used as an anesthetic.
3. Used as refrigerants, solvents, extractants, catalysts and stabilizers for polymers. [24]

 微信扫一扫打赏
微信扫一扫打赏

