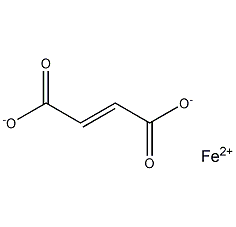
Structural formula
| Business number | 03TK |
|---|---|
| Molecular formula | C4H2FeO4 |
| Molecular weight | 169.90 |
| label |
(E)-2-Ferrous butenedioate, Ferrous fumarate, Rich in blood iron, Ferrous fumarate, iron butenedioate, Ferrous fumarate, food additives, Nutrition supplements |
Numbering system
CAS number:141-01-5
MDL number:MFCD00058315
EINECS number:205-447-7
RTECS number:LT1950000
BRN number:None
PubChem number:24894887
Physical property data
1. Physical property data:
1. Properties: orange-red to reddish-brown powder, odorless and tasteless.
2. Density (g/mL, 25/4℃): 2.435
3. Melting point (℃): >280°C
4. Dissolution Properties: Solubility in water at 25°C is 0.14g/100ml, and in ethanol is less than 0.01g/100ml
Toxicological data
2. Toxicology data:
1. Acute toxicity: Rat oral LD50: 3850 mg/kg;
Rat intraperitoneal LD50: 185 mg/kg;
Rat subcutaneous LD50: 500 mg/kg;
Mouse oral LD50: 1570 mg/kg;
Mouse intraperitoneal LD50: 480 mg/kg.
Ecological data
3. Ecological data:
Usually it is not harmful to water. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
None yet
Compute chemical data
IV. Calculated chemical data:
1. Number of hydrogen bond donors: 0
2. Number of hydrogen bond acceptors: 4
3. Number of rotatable chemical bonds: 0
4. Topological molecular polar surface area (TPSA): 80.3
5. Number of heavy atoms: 9
6. Surface charge : 0
7. Complexity: 108
8. Number of isotope atoms: 0
9. Determine the number of atomic stereocenters: 0
10. The number of uncertain atomic stereocenters: 0
11. The number of determined chemical bond stereocenters: 0
12. The number of uncertain chemical bond stereocenters: 0
13. Number of covalent bond units: 2
Properties and stability
Stable under normal temperature and pressure.
Storage method
Seal and dry�Keep away from oxidizing agents.
Synthesis method
1. Sodium enedioate is obtained by replacing it with ferrous sulfate. Heat the water to boiling, add fumaric acid with stirring, and add sodium carbonate in portions until pH=6.5-6.7. Stir thoroughly and filter while hot to obtain sodium fumarate solution. Add ferrous sulfate solution, stir and heat to reflux for 3-4 hours, cool and crystallize, and filter. The filter cake is washed with distilled water until the sulfate radical meets the requirements, and dried to obtain ferrous fumarate with a yield of 83%.
2. Obtained from the reaction of fumaric acid and iron filings. 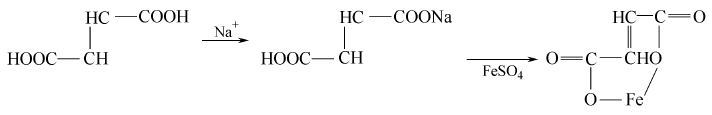
3. Add 5g fumaric acid to 100mL boiling water, and add carbonic acid Neutralize with sodium to pH 6.5-6.7; filter, add a solution of 13g ferrous sulfate and 100mL water to the obtained disodium fumarate solution, and heat, stir and reflux (100°C) for 3-4 hours; finally cool and crystallize , filter, wash and dry to obtain 6.2-6.6g of reddish brown powder ferrous fumarate, with a yield of 85%-90%.
4. Add 5g fumaric acid to 100mL boiling water, and neutralize it with sodium carbonate to a Ph value of 6.5~6.7; filter, and add 13g ferrous sulfate and 100mL to the obtained disodium fumarate solution. The solution in water is heated, stirred and refluxed (100°C) for 3 to 4 hours; finally cooled, crystallized, filtered, washed and dried to obtain 6.2 to 6.6 g of reddish brown powder ferrous fumarate, with a yield of 85% to 90%.
C4H4O4+Na2CO3→C4H2O4Na2+H2O+CO2↑
FeSO4+Na2C4H2O4→Na2SO4+FeC4H2O4
Purpose
1. As a food iron fortifier, ferrous fumarate has better absorption effect than inorganic iron. Our country stipulates that it can be used for table salt and candies, and the usage amount is 1800~3600mg/kg; in high-iron cereals and their products (daily limit of 50g of such foods), it is 520~580mg/kg; in dairy products and infant foods, it is 520~580mg/kg. 180~300mg/kg; in cereals and their products, 70~150mg/kg; in beverages, 30~60mg/kg.
2. Anti-anemia drugs are used to treat iron deficiency anemia and are also widely used in food and feed additives.
3. As a food iron fortifier, ferrous fumarate has better absorption effect than inorganic iron. Our country stipulates that it can be used in table salt and candies, and the usage amount is 1800-1600mg/kg; in high-iron cereals and their products (daily limit of 50g of such foods), it is 520-580mg/kg; in dairy products and infant foods, it is 520-580mg/kg. 180-300mg/kg; in cereals and their products, 70-150mg/kg; in beverages, 30-60mg/kg.
4. Nutritional supplements (iron fortifiers).
5. Ferrous fumarate is an anti-anemia drug. Anemia is divided into iron deficiency anemia, megaloblastic anemia and aplastic anemia according to different causes. Ferrous fumarate is used to treat iron deficiency anemia. It contains high iron content, is well absorbed, and is difficult to be oxidized into ferric iron. , the serum iron value rises quickly after taking it and remains stable. The oral LD50 for mice is 480 mg/kg.

 微信扫一扫打赏
微信扫一扫打赏

