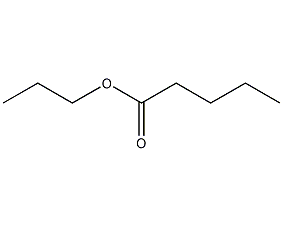
Structural formula
| Business number | 03TQ |
|---|---|
| Molecular formula | C8H16O2 |
| Molecular weight | 144.21 |
| label |
1-Propyl pentanoate, CH3(CH2)3COOCH2CH2CH3, spices, aliphatic compounds |
Numbering system
CAS number:141-06-0
MDL number:None
EINECS number:205-452-4
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Properties: colorless and transparent liquid with fruity aroma.
2. Boiling point (ºC, 101.3kPa): 167.5
3. Relative density (g/mL, 20/4ºC): 0.8699
4. Refraction Rate (20ºC): 1.4065
5. Flash point (ºC): 50
6. Solubility: Miscible with ethanol and ether, slightly soluble in water.
7. Relative density (25℃, 4℃): 0.809286.1
8. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -4851.8
9. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -583.0
Toxicological data
Slightly irritating to eyes and skin.
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 40.88
2. Molar volume (cm3/mol): 164.0
3. Isotonic specific volume (90.2K ): 375.1
4. Surface tension (dyne/cm): 27.3
5. Polarizability (10-24cm3): 16.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 89.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure. Incompatible materials: strong oxidants, strong acids, and strong bases. Flammable, easily burns when exposed to open flames or high heat.
Storage method
Store in a dry and ventilated place.
Synthesis method
1. Obtained from the esterification of valeric acid and propanol.
2.Preparation method:
Into a reaction bottle equipped with a stirrer and a reflux condenser, add 25.5g (0.25mol) of acid (2), 30g (0.5mol) of dry propanol, and anhydrous benzene 50 mL, 10 g of concentrated sulfuric acid, reflux and react for 36 hours under stirring. After the reaction is completed, pour 250 mL of water, separate the organic layer, and extract the aqueous layer with diethyl ether. The organic layers were combined, washed with saturated sodium carbonate solution and water in sequence, and dried over anhydrous sodium sulfate. After the solvent was evaporated, distillation was continued, and the fraction at 163-164°C was collected to obtain 28 g of compound (1) with a yield of 78%. [1]
Purpose
Used as solvent, spice preparation, and organic synthesis intermediate.

 微信扫一扫打赏
微信扫一扫打赏

