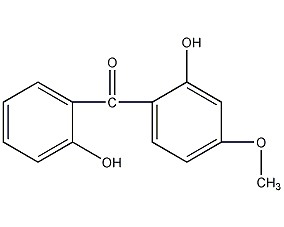
Structural formula
| Business number | 03MZ |
|---|---|
| Molecular formula | C14H12O4 |
| Molecular weight | 244.24 |
| label |
2,2′-dihydroxy-4-methoxybenzophenone, 2-Hydroxy-4-methoxyphenyl-2-hydroxyphenylmethyleneone, UV absorber UV-24, 2,2′-hydroxy-4-methoxybenzophenone, 2,2′-Dihydroxy-4-methoxybenzophenone, CH3OC6H3(OH)COC6H4OH, ultraviolet absorbent UV-24, cyasorb UV-24, UV24, UV absorber |
Numbering system
CAS number:131-53-3
MDL number:MFCD00002218
EINECS number:205-026-8
RTECS number:DJ1049500
BRN number:None
PubChem number:24859402
Physical property data
1. Properties: light yellow powder
2. Density (g/mL, 25℃): 1.382
3. Melting point (℃): 69
4. Boiling point (ºC, 0.133kpa): 170~175℃
5. Freezing point (℃): 68
6. Solubility: Solubility at 25°C (g/100g solvent): benzene 46.6, n-hexane 2.3, 95% ethanol 21.4, carbon tetrachloride 22.2, methyl ethyl ketone 55.3, plasticizer DOP 31.1, Insoluble in water.
Toxicological data
1. Mutagenicity: Salmonella gene mutation: 12500ug/L
Salmonella gene mutation: 3ug/plate
Mouse gene mutation: 32ug/plate
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 66.48
2. Molar volume (cm3/mol): 188.4
3. Isotonic specific volume (90.2K ): 513.6
4. Surface tension (dyne/cm): 55.2
5. Polarizability (10-24cm3): 26.35
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 24
6. Topological molecule polar surface area 66.8
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 292
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain chemical bondsNumber of structural centers: 0
15. Number of covalent bond units: 1
Properties and stability
Non-flammable, non-corrosive and non-toxic.
Storage method
Packaged in cardboard drums lined with plastic bags. This product is non-flammable, non-corrosive and has good storage stability.
Synthesis method
(1) Synthesis of salicylic acid chloride The reaction process uses an acid-resistant stirred reactor. First, put 5 parts of salicylic acid crystals into the kettle, add 15 parts of chlorobenzene, stir to dissolve, then add 0.03 parts of anhydrous aluminum trichloride, control the temperature between 20 and 40°C, and slowly add chlorine dropwise while stirring. Dissolve 4.5 parts of sulfoxide and proceed with the reaction. The hydrogen chloride and sulfur dioxide gas generated by the reaction undergo necessary absorption treatment. When the reaction no longer produces hydrogen chloride, it is the end point of the reaction, and the reaction to generate salicylic acid chloride is completed.
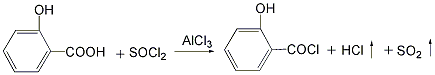
(2 ) Synthesis of 2,2′,4-trihydroxybenzophenone: Heat salicylic acid chloride under stirring. When the temperature rises to 80°C, add 3 parts of resorcinol and 0.07 parts of anhydrous pyridine to carry out the reaction. The hydrogen chloride gas produced by the reaction must be treated as necessary. The reaction temperature can be controlled at about 100°C and continued under stirring until only a small amount or no hydrogen chloride gas is released. The reaction solution is filtered, distilled, distilled under reduced pressure, cooled, crystallized, washed with alcohol, and dried to obtain 2,2’,4-trihydroxybenzophenone.
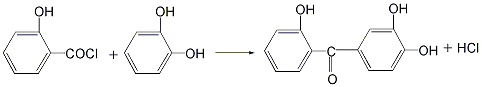
(3) Ultraviolet absorber UV Synthesis of -24: 2,2′,4-trihydroxybenzophenone and dimethyl sulfate are reacted under stirring and the temperature is controlled below 30°C for 2 to 4 hours. After the reaction is completed, the finished product is obtained by standing for stratification, vacuum distillation, cooling, activated carbon decolorization, suction filtration, concentration, cooling, crystallization, centrifugation, and low-temperature drying.
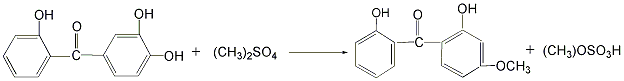
Purpose
This product is a UV absorber, suitable for many plastics such as polyvinyl chloride, ABS resin, acrylic resin, polyurethane, melamine resin, cellulose resin, etc. This product is benzophenone containing two ortho-positioned hydroxyl groups. It has the strongest ability to absorb ultraviolet rays and has a strong absorption effect on ultraviolet rays with a wavelength of 330 to 370nm. The disadvantage is that it also absorbs part of visible light, making the product slightly yellow. This product has good compatibility with resin, and the general dosage is 0.25% to 3%. It also has good light stabilizing effect in paint.
It is a strong UV absorber, suitable for materials such as polyvinyl chloride, ABS resin, acrylic resin, polyurethane, melamine resin, cellulose resin, etc. It has good compatibility with the resin base material, and the general dosage is 0.25% to 3%.

 微信扫一扫打赏
微信扫一扫打赏

