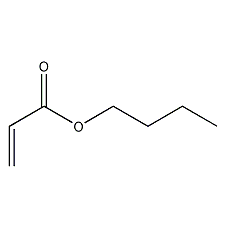
Structural formula
| Business number | 03TY |
|---|---|
| Molecular formula | C7H12O2 |
| Molecular weight | 128.17 |
| label |
2-Butyl acrylate, n-butyl acrylate, acrylic acid n-butyl ester, n-Butyl acrylate, aliphatic compounds |
Numbering system
CAS number:141-32-2
MDL number:MFCD00009446
EINECS number:205-480-7
RTECS number:UD3150000
BRN number:1749970
PubChem number:24845988
Physical property data
1. Properties: Colorless and transparent liquid with strong fruity aroma. [1]
2. Melting point (℃): -64.6[2]
3. Boiling point (℃): 145~149[3]
4. Relative density (water=1): 0.90[4]
5. Relative vapor density (air=1): 4.42[5]
6. Saturated vapor pressure (kPa): 0.43 (20℃)[6]
7. Heat of combustion (kJ/mol): -4073.2[7]
8. Critical temperature (℃): 327[ 8]
9. Critical pressure (MPa): 2.94[9]
10. Octanol/water partition coefficient: 2.38[10]
11. Flash point (℃): 36[11]
12. Ignition temperature (℃) :267~292[12]
13. Explosion upper limit (%): 9.9[13]
14. Explosion Lower limit (%): 1.3[14]
15. Solubility: Insoluble in water, miscible in ethanol and ether. [15]
16. Viscosity (mPa·s, 20ºC): 0.90
17. Viscosity (mPa·s, 25ºC): 0.81
18. Viscosity (mPa·s, 40ºC): 0.70
19. Flash point (ºC, open): 47
20. Flash point (ºC, closed ): 41
21. Vapor pressure (kPa, 0ºC): 0.14
22. Vapor pressure (kPa, 20ºC): 0.44
23. Vapor pressure (kPa, 50ºC): 2.82
24. Vapor pressure (kPa, 100ºC): 21.9
25. Relative density (25℃, 4℃): 0.8934
26. Refractive index at room temperature (n25): 1.4156
27. Solubility parameter (J·cm-3)0.5 : 17.121
28. van der Waals area (cm2·mol-1): 1.131×1010
29. van der Waals volume (cm3·mol-1): 80.170
Toxicological data
1. Acute toxicity[16]
LD50: 900mg/kg (rat oral); 5880mg/kg (mouse Oral); 1800mg/kg (rabbit transdermal)
LC50: 14305mg/m3; 2730ppm (rat inhalation, 4h)
2. Irritation [ 17]
Rabbit transdermal: 10mg (24h), mild stimulation (open stimulation test).
Rabbit eye: 50mg, mild irritation.
3. Others[18] Minimum toxic concentration by inhalation in rats (TCLo): 135ppm (6h) (gestation 6~15d), implanted Mortality rate increased afterwards.
Ecological data
1. Ecotoxicity[19]
LC50: 23mg/L (The chemical method is the latest method for producing acrylic acid and its esters. Raw material consumption quota: acrylic acid 770kg/t, n-butanol 610kg/t.
5. Preparation method:
In a reaction bottle equipped with a Webster fractionating column (with distillation device), a stirrer, and a ventilation tube, add 371g (5.0 mol) of n-butanol. ), 861g (10.0mol) of methyl acrylate (2), 20g of hydroquinone and 10g of p-toluenesulfonic acid, add carbon dioxide gas, stir and heat to reflux in an oil bath. The first thing to evaporate is the azeotrope of methanol-methyl acrylate (62~63°C), and the temperature of the distillation head is controlled not to exceed 65°C. When the methanol production is very slow in about 8 to 10 hours, evaporate excess methyl acrylate under reduced pressure, and then collect the 39°C/1.33kPa fraction under reduced pressure to obtain n-butyl acrylate ① (1) 500~600g, yield 78%~94%. Note: ① This method uses an excess of ester for transesterification reaction, and the ester can be recycled after recovery. This method can be used to synthesize the following various esters (Table I-9-1, page 485 of the reference book). [28]
Purpose
1. Acrylic acid and its esters are widely used in industry. During use, acrylic esters are often polymerized into polymers or copolymers. Butyl acrylate (and methyl ester, ethyl ester, 2-ethylhexyl) is a soft monomer and can be combined with various hard monomers such as methyl methacrylate, styrene, acrylonitrile, vinyl acetate, etc., and has functional Monomers such as hydroxyethyl (meth)acrylate, hydroxypropyl ester, glycidyl ester, (meth)acrylamide and its derivatives are copolymerized, cross-linked, grafted, etc. to produce more than 200-700 kinds of acrylic resins Products (mainly emulsion type, solvent type and water-soluble type) are widely used in coatings, adhesives, acrylic fiber modification, plastic modification, fiber and fabric processing, paper treatment agents, leather processing, acrylic rubber and many other aspects.
2. Soft monomers used in the manufacture of acrylic solvent-based and emulsion-based adhesives can be homopolymerized, copolymerized and grafted. It is widely used in adhesives, coatings, plastic modification, fiber processing, paper impregnation, leather processing, etc. Copolymerized with vinyl acetate to produce building sealants to improve toughness and water resistance. As the second monomer and methyl methacrylate grafted with SBS, an SBS graft adhesive with strong initial adhesion and high bonding strength can be produced. It can be copolymerized with hard monomers such as styrene, acrylonitrile, vinyl acetate, methyl methacrylate, and functional monomers such as hydroxyethyl acrylate, hydroxypropyl acrylate, glycidyl ester, and methacrylamide to produce emulsion type, Solvent-based and water-soluble products. Can also be used as a solvent.
3. High molecular polymer monomer. Mainly used as fibers, rubber, plastics, coatings, adhesives, textile auxiliaries, and also as a treatment agent for leather and paper.
4. Used as an organic synthesis intermediate for the production of resins, coatings, adhesives, emulsifiers, etc. [27]

 微信扫一扫打赏
微信扫一扫打赏

