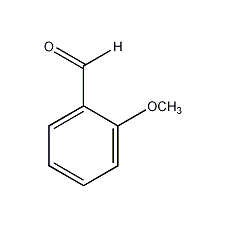
Structural formula
| Business number | 03PW |
|---|---|
| Molecular formula | C8H8O2 |
| Molecular weight | 136.15 |
| label |
Salicylaldehyde methyl ether; 2-methoxybenzaldehyde; o-methoxybenzaldehyde, aromatic compounds |
Numbering system
CAS number:135-02-4
MDL number:MFCD00003308
EINECS number:205-171-7
RTECS number:BZ2610000
BRN number:606301
PubChem number:24883419
Physical property data
1. Character: colorless prismatic crystal
2. Melting point (ºC): 37~38
3. Boiling point (ºC): 236
4. Boiling point (ºC, 0.20kpa): 70~75
5. Relative density (20℃, 4℃): 1.10451
6 .Relative density (25℃, 4℃): 1.025131
7. Refractive index: 1.5597
8. Flash point (℃): 117
9. Solubility: soluble in ethanol, ether, benzene, insoluble in water.
10. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -4025.0
11. Crystal phase standard claim heat ( Enthalpy) (kJ·mol-1): -266.4
Toxicological data
1. Skin/eye irritation data
Rabbit skin contact: 500mg/24H moderate reaction;
2. Acute toxicity data:
Rat Oral LD50: 2500mg/kg; Mouse oral LD50: 2400mg/kg; Rabbit skin LD50: >5mg/kg;
3. Mutagenicity data:
Human lymphocytes Sister chromatid changes: 125umol/L.
Ecological data
None
Molecular structure data
1. Molar refractive index: 39.68
2. Molar volume (cm3/mol): 125.1
3. Isotonic specific volume (90.2K ): 309.0
4. Surface tension (3.0 dyne/cm): 37.2
5. Polarizability (0.5 10-24cm 3): 15.73
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 112
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It turns yellow when reacting with sulfuric acid.
Storage method
Stored in a well-ventilated, cool place.
Synthesis method
It is produced by the methylation reaction of salicylaldehyde and dimethyl sulfate. Prepare 3kg sodium hydroxide into a 30% aqueous solution, add it to a solution containing 12.2kg salicylaldehyde and 80L water while stirring, and heat to boiling. Slowly add 12.9kg of dimethyl sulfate, keep the reaction solution refluxing for about 3 hours, then continue refluxing for 2-3 hours, and cool the oil layer.�Wash with 5% sodium hydroxide solution, then wash with water until the pH is 8, add anhydrous potassium carbonate and dry. Filter out the desiccant and then distill under reduced pressure to collect the 120°C (2.0kPa) fraction, which is o-methoxybenzaldehyde. The methylation reaction can also be carried out in the solvent toluene, with a yield of 60%.
Purpose
Organic synthetic raw materials for the production of medicines and spices. It is also a general organic reagent.

 微信扫一扫打赏
微信扫一扫打赏

