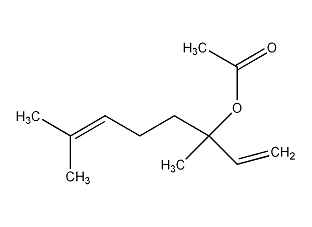
Structural formula
| Business number | 038C |
|---|---|
| Molecular formula | C12H20O2 |
| Molecular weight | 196.29 |
| label |
Linalyl acetate, galoxylate acetate, Linal (alcohol) acetate, Bergamol, Phanteine, 3,7-Dimethyl-1,6-octadien-3-yl Acetate, artificial flavors, Multifunctional solvent |
Numbering system
CAS number:115-95-7
MDL number:MFCD00008907
EINECS number:204-116-4
RTECS number:RG5910000
BRN number:1724500
PubChem number:24901214
Physical property data
1. Properties: colorless to light yellow liquid with a pleasant floral and fruity aroma, like bergamot and lavender, strong and sweet.
2. Density (g/mL, 20℃): 0.901
3. Relative vapor density (g/mL, air=1): 6.8
4. Melting point (ºC): 85
5. Boiling point (ºC, normal pressure): 220
6. Boiling point (ºC, 3.33KPa): 115-116
7. Refractive index (n20D): 1.453
8. Flash point (ºC): 90
9. Relative density (20℃, 4℃): 0.8951
10. Refractive index at room temperature (n20): 1.454421
11. Vapor pressure (mmHg, 20ºC): 0.1
12. Saturated vapor pressure (kPa, ºC): Undetermined
13 . Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion The lower limit (%, V/V) has not been determined:
19. Solubility: miscible with ethanol and ether, soluble in mineral oil, animal and vegetable oils, insoluble in water
Toxicological data
1. Irritation: Rabbit percutaneous standard Drez eye dye test: 100mg/24H severe irritation.
Guinea pig percutaneous standard Drez eye dye test: 100mg/24H moderate irritation.
2. Acute toxicity: Rat oral LD50: 13934mg/kg
Mouse oral LD50: 12g/kg
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 59.00
2. Molar volume (cm3/mol): 218.6
3. Isotonic specific volume (90.2K): 501.7
4. Surface tension (dyne/cm): 27.7
5. Polarizability (10-24cm3): 23.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 237
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Soluble in ethanol, ether, diethyl phthalate, benzyl benzoate, non-volatile oil and mineral oil, slightly soluble in propylene glycol, insoluble in water and glycerin.
2. Found in burley tobacco leaves.
3. Natural products are widely found in essential oils such as lavender, bergamot, lemon, basil, jasmine, ylang-ylang, rose, camphor, orange blossom, orange leaf and basil.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Linalyl acetate exists in natural essential oils such as bergamot, orange leaf, neroli, lavender, and linoleum. Therefore, linalyl acetate can be isolated from natural essential oils by vacuum fractionation. But industrially it is generally prepared by the reaction of acetic anhydride and linalool.
Adding linalool (a complex catalyst formed by phosphoric acid and acetic anhydride) into the mixture of acetic anhydride and phosphoric acid allows the reaction to proceed at a lower temperature. After the esterification is completed, wash with water and saturated brine until neutral. After adding anhydrous sodium carbonate to dehydrate and dry, the product was obtained by fractionation under reduced pressure, with a purity of >95%. The obtained product has a high ester content and a positive aroma.
2.Ketone method
Reaction Put 100 parts (weight ratio) of linalool and 1 part of p-toluene sulfonate into the kettle, then pass ketene into the reaction kettle, and maintain the temperature at 20ºC. When ketene is absorbed by linalool to 83% mole, The conversion of linalool into ester is 73.5%. The crude product was fractionated under reduced pressure to obtain the pure product.
3. Tobacco: BU, 18.
Purpose
1. Suitable for perfume, hair spray, cosmetics and soap. It is an important raw material for preparing fragrances such as bergamot, orange leaf, lavender, and mixed lavender. It is also one of the base spices used in the preparation of jasmine, orange blossom and other fragrances. Used as a coordinating modifier for sweet and fresh floral scents such as ylang-ylang to enhance the fresh fruity aroma. It can also be used in small amounts in food flavors.
2.Linalyl acetate is an edible flavor allowed in my country. It can be used to prepare fruity flavors such as pineapple, citrus, and peach. The dosage should be as per normal production need. In chewing gum 13mg/kg; in candy 11mg/kg; in baking 8.9mg/kg in baked food; 3.8mg/kg in cold drinks.

 微信扫一扫打赏
微信扫一扫打赏

