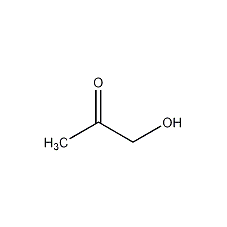
Structural formula
| Business number | 038G |
|---|---|
| Molecular formula | C3H6O2 |
| Molecular weight | 74.08 |
| label |
Hydroxyacetone, 1-Hydroxy-2-propanone, acetylmethanol, Acetol, 1-Hydroxy-2-propanone, 2-Oxopropanol, stabilizer, Nitrocellulose solvent, Peptide synthesis protectant |
Numbering system
CAS number:116-09-6
MDL number:MFCD00004669
EINECS number:204-124-8
RTECS number:UC2800000
BRN number:605368
PubChem number:24848238
Physical property data
1. Properties: colorless, irritating liquid with fragrance. Found in beer, tobacco and honey.
2. Density (g/mL, 20/4℃): 1.0804
3. Melting point (ºC): -17
4. Boiling point (ºC ): 44.415℃ (decomposition)
5. Boiling point (ºC, 20KPa): 145~146
6. Boiling point (ºC, 2.4KPa) :54
7. Refractive index (20ºC): 1.4295
8. Flash point (ºC): 56
9. Solubility: soluble in water, Ethanol, ether.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2200mg/kg
Ecological data
Slightly harmful to water. Methyl ketone is of low toxicity. When the vapor is inhaled, it can irritate the mucous membranes of the eyes and nostrils and cause headaches.
Molecular structure data
1. Molar refractive index: 17.51
2. Molar volume (cm3/mol): 72.6
3. Isotonic specific volume (90.2K ): 173.3
4. Surface tension (dyne/cm): 32.3
5. Polarizability (10-24cm3): 6.94
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.7
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 4
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 40.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable and easy to polymerize into dimers. In addition to having hydroxyl and carbonyl properties, it is also easily oxidized by cuprous oxide and forms uracil with phenylhydrazine. Avoid contact with strong oxidants and moisture. Flammable and easy to absorb moisture. Avoid inhalation of vapor and smoke when using, and avoid contact with eyes and skin.
2. It is flammable and easy to absorb moisture. When using, avoid inhaling the vapor and smoke of this product, and avoid contact with eyes and skin.
3. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and mainstream smoke.
4. Found in beer and honey.
Storage method
1. Store in a cool, ventilated warehouse. Store at 0-4ºC. Keep away from fire, heat and water sources. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. It is produced by catalytic dehydrogenation of propylene glycol or the reaction of bromoacetone and potassium formate to form acetol formate, which is then hydrolyzed. It can also be obtained by catalytic dehydrogenation of glycerol or propylene glycol.
2.Carry out rectification, ultra-clean filtration, and ultra-clean packaging of industrial butanone to obtain BV-III grade butanone.
3. Preparation method:
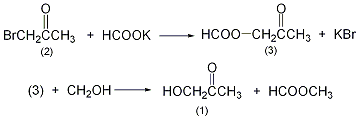
In a reaction bottle equipped with a stirrer and reflux condenser, add 500 mL of methanol (water content <0.2%), slowly add 110 g (1.2 mol) of potassium formate while stirring, stir thoroughly and add Make it dissolve. After cooling, slowly add brominated acetone ①(2) 140g (1mol), and KBr precipitates soon. Reflux reaction for 15h. Cool in an ice-salt bath, filter out the potassium bromide, fractionate and reflux methanol and methyl formate, and then fractionate under reduced pressure to collect the 30-40°C/5-1.6kPa fraction to obtain hydroxyacetone ②(1 )17g, yield 45%. Note: ① Bromoacetone has strong tear-inducing and irritating effects, so special attention should be paid when using it. ②Hydroxyacetone is unstable and easy to polymerize, but it is easy to prepare 50% methanol and can last for more than one year. [1]
Purpose
1. Used to prepare drugs, spices, dyes, etc. Methanol can be added as a stabilizer during storage. Organic synthesis intermediates, solvents for nitrocellulose, and protective agents for peptide synthesis.
2.Electronic pure ethyl ketone is used as a developer and cleaning agent in the photolithography process of integrated circuits in the electronic industry. MOS level is mainly used for discrete devices and large and medium-scale integrated circuits, and BV-III level is mainly used for the production of very large-scale integrated circuits.

 微信扫一扫打赏
微信扫一扫打赏

