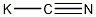
Structural formula
| Business number | 03YR |
|---|---|
| Molecular formula | KCN |
| Molecular weight | 65.12 |
| label |
Mountain milk potassium, Potassium cyanide solution, masking agent, compounding agent, pesticides, photographic fixative, Raw materials for printed circuit board electroplating |
Numbering system
CAS number:151-50-8
MDL number:MFCD00011397
EINECS number:205-792-3
RTECS number:TS8750000
BRN number:3593645
PubChem number:24881807
Physical property data
1. Properties: white crystal or powder, easy to deliquesce. [12]
2. pH value: 11 (0.1mol/L aqueous solution) [13]
3. Melting point (℃): 634.5[14]
4. Relative density (water=1): 1.52[15]
5. Octanol/water partition coefficient: -1.69[16]
6. Solubility: easily soluble in water, ethanol, glycerol, slightly soluble in methanol and sodium hydroxide aqueous solution. [17]
Toxicological data
1. Acute toxicity[18] LD50: 5mg/kg (rat oral)
2. Irritation No data yet
3. Mutagenicity [19] DNA inhibition: mouse lymphocytes 1mmol/L. Cytogenetic analysis: human lymphocytes 70mg/L (24h). DNA damage: rat liver 300 μmol/L.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No data yet
4. Other harmful effects[20] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: not available
2. Molar volume (cm3/mol): not available
3. etc. Zhang specific volume (90.2K): None available
4. Surface tension (dyne/cm): None available
5. Dielectric constant: None available
6. Polarizability (10-24cm3): None available
7. Single isotope mass: 64.966781 Da
8. Nominal mass: 65 Da
9. Average mass: 65.1157 Da
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 23.8
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 12.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters20508/201205081426245946.jpg” alt=”” />
Sometimes potassium cyanide participates in the reaction, but the carbon chain of the product is not necessarily extended. For example, in the presence of potassium cyanide, ammonia can react with carboxylic acid esters The reaction generates hydroxamic acid (formula 3)[7].
AsacatalystInadditiontobeingareactant,potassiumcyanidecanalsobeusedasacatalyst.Forexample,itcancatalyzecertainreactionstoformC-Cbonds(formula4)[8].
Potassium cyanide and 18-crown ether-6 form a catalytic system, which has a strong catalytic effect and can be used for the coupling reaction of carbon-carbon bonds (Formula 5)[9], the reaction mechanism is shown in Formula 6.
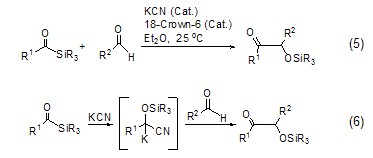
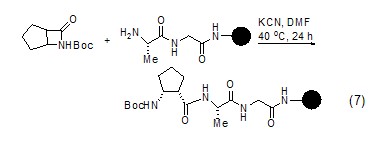
Using potassium cyanide as a catalyst can also be used to form amide bonds, thereby extending the peptide chain (Formula 7)[10].
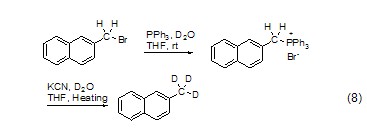
Halogenated hydrocarbons react with PPh3 to form salts, Hydrolysis in the presence of KCN and heavy water can give high yield of deuterated products (Formula 8)[11].
7. Used for refining gold, silver and other precious metals, quenching, electroplating, and preparing analytical reagents, organic nitriles, and medicine , pesticides, etc. [26]

 微信扫一扫打赏
微信扫一扫打赏

