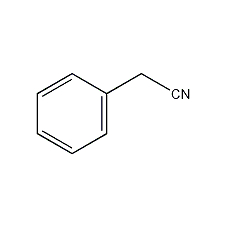
Structural formula
| Business number | 03SX |
|---|---|
| Molecular formula | C8H7N |
| Molecular weight | 117 |
| label |
benzyl cyanide, Alpha-cyanotoluene, benzyl cyanide, Benzyl cyanide, α-Tolunitrile, Benzeneacetonitrile, 2-Phenylacetonitrile, synthetic raw materials |
Numbering system
CAS number:140-29-4
MDL number:MFCD00001894
EINECS number:205-410-5
RTECS number:AM1400000
BRN number:385941
PubChem number:24848087
Physical property data
1. Properties: colorless oily liquid with aromatic odor. [1]
2. Melting point (℃): -23.8[2]
3. Boiling point (℃): 233.5[3]
4. Relative density (water = 1): 1.02[4]
5. Saturated steam Pressure (kPa): 0.0123 (25℃)[5]
6. Heat of combustion (J/mol): -4278.2[6]
7. Octanol/water partition coefficient: 1.56[7]
8. Flash point (℃): 101[8] sup>
9. Explosion upper limit (%): 7[9]
10. Explosion lower limit (%): 1.1[10]
11. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol and ether. [11]
12. Refractive index (20ºC): 1.5230
13. Viscosity (mPa·s, 25ºC): 1.956
14. Conductivity (S/m): <0.5×10-7
Toxicological data
1. Acute toxicity[12]
LD50: 270mg/kg (rat oral); 45.5mg/kg (mouse Oral); 270mg/kg (rabbit transdermal)
LC50: 430mg/m3 (rat inhalation, 2h)
2 . Irritation [13] Rabbit transdermal: 500mg (24h), mild irritation.
3. Sensitization [14] Skin sensitization
Ecological data
1. Ecotoxicity[15] EC50: 1.2~1.51mg/L (photobacteria, Microtox toxicity test)
2. Biodegradability[16] MITI-I test, initial concentration 100mg/L, sludge concentration 30mg/L, 77% degradation after 2 weeks .
3. Non-biodegradability[17] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 7.8d (theoretical).
4. Other harmful effects [18] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 35.71
2. Molar volume (cm3/mol): 115.6
3. Isotonic specific volume (90.2K ): 292.1
4. Surface tension ��3.0 dyne/cm): 40.7
5. Polarizability (0.5 10-24cm3): 14.15
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 23.8
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 114
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has the general chemical properties of nitrile. Sodium thiosulfate has a detoxifying effect on this product.
2. Stability[19] Stable
3. Incompatible substances[20] Strong oxidizing agent, strong reducing agent, strong acid, strong alkali
4. Polymerization hazard[21] No polymerization
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the reaction of benzyl chloride and sodium cyanide. The reaction was carried out in alcohol solvent with dimethylamine as catalyst. The reaction temperature is 80-100°C. After the crude product of phenylacetonitrile is obtained through the reaction, the finished product is obtained by distillation under reduced pressure. The purity of the refined product can reach 99%, and the yield is about 90%. Raw material consumption quota: benzyl chloride (96%) 1270kg/t, sodium cyanide 490kg/t, ethanol (95%) 544kg/t, dimethylamine (30%) 17kg/t
Refining method : The phenylacetonitrile is shaken vigorously with an equal amount of 50% sulfuric acid at 60°C to remove the isonitrile, and then the phenylacetonitrile is separated, washed with sodium bicarbonate solution and saturated brine, dried and distilled under reduced pressure. Other purification methods include passing phenylacetonitrile through a column filled with activated alumina and then distilling it in the presence of skeleton nickel.
Purpose
1. Used as gas chromatography stationary liquid for separation of gaseous hydrocarbons and halogenated hydrocarbons. It is also used as an intermediate for pesticides (such as phoxim), medicines (such as phenobarbital), dyes, etc.
2. Used as intermediates for medicines, pesticides, dyes and spices. Products produced using phenylacetonitrile include phenylacetic acid, phenylacetone, 2-cyanobenzyl oxime, 3-phenyl-5-chloromethylbenzisoxazole, methyl phenylacetate, phoxim emulsifiable concentrate, etc.
3. Used in organic synthesis. [23]

 微信扫一扫打赏
微信扫一扫打赏

