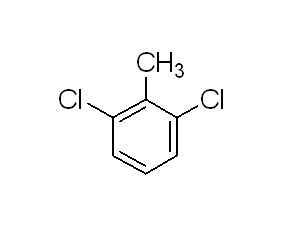
Structural formula
| Business number | 03A0 |
|---|---|
| Molecular formula | C7H6Cl2 |
| Molecular weight | 161 |
| label |
None yet |
Numbering system
CAS number:118-69-4
MDL number:MFCD00000576
EINECS number:204-269-7
RTECS number:None
BRN number:1907194
PubChem number:24869594
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): 2.8[2]
3. Boiling point (℃): 196 ~203[3]
4. Relative density (water = 1): 1.25[4]
5. Relative Vapor density (air = 1): 5.6[5]
6. Octanol/water partition coefficient: 4.25[6]
7. Flash point (℃): 82[7]
8. Ignition temperature (℃): >600[8]
9. Solubility: Insoluble in water, soluble in chloroform. [9]
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradable[10] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 12d (theoretical).
4. Bioconcentration[11] BCF: 1100 (theory)
Molecular structure data
1. Molar refractive index: 40.86
2. Molar volume (cm3/mol): 129.6
3. Isotonic specific volume (90.2K ): 316.6
4. Surface tension (dyne/cm): 35.6
5. Polarizability (10-24cm3): 16.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 82.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. There is a danger of combustion and explosion when exposed to open flames, high heat, or oxidants.
2. Stability[12] Stable
3. Incompatible substances[13] Strong oxidizing agent, strong alkali
4. Conditions to avoid contact[14] Heating
5. Polymerization hazard[15] No polymerization
6. Decomposition products[16] Hydrogen chloride
Storage method
Storage Precautions[17] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It is obtained by diazotization and substitution of 3-chloro-2-methylaniline.
Purpose
1. Used as solvent and organic synthesis intermediate. It is the basic raw material of diclofenac and penicillin. It can also be used to synthesize many important medicines, such as 6-acetylbenzothiazolones.
2. Used as raw material for organic synthesis and used to manufacture dyes, herbicides, etc. [18]

 微信扫一扫打赏
微信扫一扫打赏

