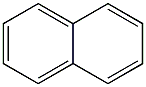
Structural formula
| Business number | 023A |
|---|---|
| Molecular formula | C10H8 |
| Molecular weight | 128 |
| label |
Naphthalene, Naphthalin, Naphthene, Tar camphor, aromatic compounds |
Numbering system
CAS number:91-20-3
MDL number:MFCD00001742
EINECS number:202-049-5
RTECS number:QJ0525000
BRN number:1421310
PubChem number:24888246
Physical property data
1. Properties: white volatile crystals with a mild aromatic odor, while crude naphthalene has the odor of coal tar. [1]
2. Melting point (℃): 80.1[2]
3. Boiling point (℃): 217.9 [3]
4. Relative density (water = 1): 1.16[4]
5. Relative vapor density (Air=1): 4.42[5]
6. Saturated vapor pressure (kPa): 0.0131 (25℃)[6]
7. Heat of combustion (kJ/mol): -4983[7]
8. Critical temperature (℃): 475.2[8]
9. Critical pressure (MPa): 4.05[9]
10. Octanol/water partition coefficient: 3.01~3.59[10]
11. Flash point (℃): 78.9[11]
12. Ignition temperature (℃): 526[12]
13. Explosion upper limit (%): 5.9 (steam) [13]
14. Explosion Lower limit (%): 2.5g/m3 (dust); 0.9 (steam) [14]
15. Solubility: insoluble in water , soluble in absolute ethanol, ether and benzene. [15]
16. Viscosity (mPa·s, 99.8ºC): 0.7802
17. Flash point (ºC, opening): 79
18. Flash point (ºC, closed): 78.9
19. Heat of evaporation (KJ/mol, 167.7ºC): 46.415
20. Heat of formation (KJ /mol, 25ºC, solid): 78.50
21. Heat of formation (KJ/mol, 25ºC, liquid): 96.38
22. Heat of formation (KJ/mol, 25ºC, gas ): 151.77
23. Heat of fusion (KJ/mol): 19.18
24. Specific heat capacity (KJ/(kg·K), -258ºC, constant pressure): 0.046
25. Specific heat capacity (KJ/(kg·K), 87.5ºC, constant pressure): 1.683
26. Specific heat capacity (KJ/(kg·K), 90ºC, constant pressure): 1.775
27. Boiling point rising constant: 5.80
28. Conductivity (S/m): 4.35×10-10
29. Solubility (g/L, water, 0ºC): 0.019
30. Solubility (g/L, water, 100ºC): 0.030
31. Thermal conductivity (W /(m·K),100≤t≤140 ºC): (0.1654~1.163)×10-4 t
32. Volume expansion coefficient (K– 1): 0.000853
33. Critical density (g·cm-3): 0.315
34. Critical volume (cm3·mol-1): 407
35. Critical compression factor: 0.265
36. Eccentricity factor: 0.302
37. Solubility parameter (J·cm-3)0.5: 19.188
38. van der Waals area (cm2·mol-1): 8.420×109
39. van der Waals volume (cm3·mol-1): 74.640
40. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5229.67
41. Gas phase standard claims heat (enthalpy) (kJ·molThe device should be sealed to prevent vapor powder from escaping, and the operation site should be forced to ventilate. If poisoning occurs, immediately move to a place with fresh air, drink plenty of hot water, induce vomiting, and perform artificial respiration. Severe cases should be sent to the hospital for treatment.
3. Stability[27] Stable
4. Incompatible substances[28] Strong oxidizing agents (such as chromic anhydride, chlorate and potassium permanganate, etc.)
5. Polymerization hazard[29] No aggregation
Storage method
Storage Precautions[30] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 35℃. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Separate from coal tar. Naphthalene accounts for about 8%-12% in high-temperature coal tar. Distill the coal tar, cut the kerosene, remove phenol, remove quinoline, and distill to obtain the finished naphthalene. Each ton of naphthalene consumes 10t of coal tar.
2. Produced from petroleum hydrocarbons: catalytic heavy reformed oil, catalytic cracking light cycle oil, tar by-product of cracking to produce ethylene, etc.
3. Crude naphthalene is refined with white clay to obtain refined naphthalene.
4. Static fractional crystallization method: Put the raw material industrial naphthalene into the crystallization box and quickly cool it down to 82°C, then switch to uniform cooling, and cool to 60°C at a cooling rate of 2°C/h. The first crystallized naphthalene oil rich in thiindane is discharged as a middle distillate for post-processing. Then the material in the crystallization box is heated at a rate of 4℃/h, and samples are taken every 0.5h to measure the crystallization point. According to the different crystallization points, they are discharged into the corresponding fraction tanks. In this way, 3 to 4 times of step-by-step crystallization can be achieved. Obtain higher purity refined naphthalene.
5. Falling film step-by-step crystallization method The crystallization method production process consists of product production process system, energy system, nitrogen sealing system and computer control system. The production process system uses a large cycle as the production cycle. Each large cycle contains 4 small cycles, and each small cycle contains 4 to 6 sections. Each section consists of 3 steps: crystallization, partial melting and full melting. Example of falling film crystallization operation process: The liquid industrial naphthalene sent from the industrial naphthalene device is sent into the fraction tank. When the fourth stage of crystallization operation is performed, the raw material liquid in the tank is sent to the dynamic crystallizer collection tank with a pump. The uncrystallized naphthalene oil and sweat liquid are put into a fraction tank with a lower purity, and the whole melt can be used as the raw material for the fifth stage. After six stages of crystallization and purification according to the predetermined procedure, the product refined naphthalene can be obtained. In order to improve the extraction rate of naphthalene, the thiindane-rich fraction can be sent to a static crystallizer for processing. The product obtained from the static crystallizer is returned to the corresponding fraction tank of the dynamic crystallization system, and the residual liquid can be sold as a water-reducing agent. Since the device uses both dynamic and static crystallizers, it can not only ensure a high recovery rate of naphthalene, but also reduce energy consumption.
Purpose
1. Naphthalene is the most important condensed ring hydrocarbon in industry. It is mainly used to produce phthalic anhydride; various naphthols; naphthylamine, etc., and is an intermediate for the production of synthetic resins; plasticizers; dyes; surfactants; synthesis Fibers; coatings; pesticides; medicines; spices; raw materials for rubber additives and pesticides. The use distribution of naphthalene varies from country to country. Approximately 70% is used for the production of phthalic anhydride, approximately 15% is used for dye intermediates and rubber additives, approximately 6% is used for pesticides, and approximately 4% is used for tanning agents. The United States uses a larger proportion for the production of pesticides, mainly for the production of carbaryl. Using naphthalene as raw material, various intermediates can be produced through unit operations such as sulfonation, nitration, reduction, amination, and hydrolysis. The application of refined naphthalene is still expanding. The new product “super plastic material”, naphthalene sulfonate formaldehyde condensate, can be used as a cement additive to increase the plastic deformation of concrete without reducing its strength. Demand will grow at a rate of 5-10% in the next few years.
2. Used in the manufacture of dye intermediates, camphor balls, leather and wood protective agents, etc. [31]

 微信扫一扫打赏
微信扫一扫打赏

