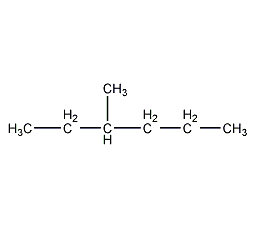
Structural formula
| Business number | 0600 |
|---|---|
| Molecular formula | C7H16 |
| Molecular weight | 100.21 |
| label |
2-Ethylpentane, 2-Ethylpentane |
Numbering system
CAS number:589-34-4
MDL number:MFCD00009408
EINECS number:209-643-3
RTECS number:None
BRN number:1718739
PubChem number:24885173
Physical property data
1. Physical property data
1. Properties: colorless flammable liquid, irritating.
2. Density (g/mL, 25/4℃): 0.687
3. Refractive index (nD20): 1.3860
4. Flash point (℃): (closed cup) -3℃
5. Melting point (℃): -119.4
6. Boiling point (ºC): 92
7. Solubility: Miscible with ether, acetone, benzene, and chloroform, soluble in ethanol, and insoluble in water.
8. Critical temperature (K): 262.05
9. Critical pressure (MPa): 2.81
10. Critical density (g·cm -3): 0.248
11. Critical volume (cm3·mol-1): 404
12. Critical compression factor: 0.256
13. Eccentricity factor: 0.322
14. Solubility parameter (J·cm-3) 0.5: 15.020
15. van der Waals area (cm2·mol-1): 1.098×1010
16. van der Waals volume (cm3·mol-1): 78.480
17. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4849.84
18. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -191.38
19. Gas phase standard entropy (J·mol-1·K-1): 426.1
20. Gas phase standard formation free energy (kJ·mol-1): 5.3
21. Gas phase standard hot melt (J·mol-1 sup>·K-1): 163.59
22. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -4814.78
23. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -226.44
24. Liquid phase standard entropy (J· mol-1·K-1): 309.6
25. Liquid phase standard free energy of formation (kJ·mol-1):-4.7
26. Liquid phase standard��Melt(J·mol-1·K-1): 218.8
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 34.43
2. Molar volume (cm3/mol) 144.4
3. Isotonic specific volume (90.2K) : 308.0
4. Surface tension (dyne/cm): 20.6
5. Polarizability (10-24cm3): 13.65
Compute chemical data
IV. Calculated chemical data:
1. Hydrophobic parameter calculation reference value (XlogP): 3.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 3
5. Topological molecular polar surface area (TPSA): 0
6. Number of heavy atoms: 7
7. Surface charge: 0
8. Complexity: 31
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters: 0
11. Uncertain number of atomic stereocenters: 1
12. Determine the number of chemical bond stereocenters: 0
13. Number of uncertain stereocenters of chemical bonds: 0
14. Number of covalent bond units: 1
Properties and stability
1. Exist in flue-cured tobacco leaves and smoke.
Storage method
2. Storage
This product should be sealed and stored in a cool place.
Synthesis method
Tobacco: FC, 40
Purpose
3. Use
Used in organic synthesis. Oil solvents. Gas chromatography standards.

 微信扫一扫打赏
微信扫一扫打赏

