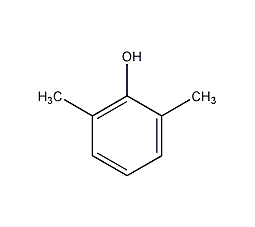
Structural formula
| Business number | 05V6 |
|---|---|
| Molecular formula | C8H10O |
| Molecular weight | 122.16 |
| label |
2,6-xylenol, 2-Hydroxy-m-xylene, 2,6-Xylenol, Photographic chemicals, phenolic solvents, aromatic compounds |
Numbering system
CAS number:576-26-1
MDL number:MFCD00002240
EINECS number:209-400-1
RTECS number:ZE6125000
BRN number:1446677
PubChem number:24862396
Physical property data
1. Properties: white crystal.
2. Boiling point (ºC, 101.3kPa): 203
3. Melting point (ºC): 45.8
4. Relative density (g/mL, 25 /25ºC, solid): 1.014
5. Relative density (g/mL, 80/4ºC): 0.983
6. Crystalline phase standard combustion heat (enthalpy) (kJ· mol-1): -4339.9
7. Refractive index (n20ºC): 1.5371d
8. Flash point ( ºC): 73
9. Heat of evaporation (KJ/mol): 44.55
10. Heat of formation (KJ/mol): 237.60
11. Combustion Heat (KJ/mol): 4342.76
12. Critical temperature (ºC): 427.8
13. Solubility: easily soluble in alcohol, ether, chloroform, benzene and alkali solutions, Slightly soluble in water
14. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -237.4
15. Gas phase standard combustion Heat (enthalpy) (kJ·mol-1): -4415.5
16. The gas phase standard claims heat (enthalpy) (kJ·mol-1 ): -161.8
17. Gas phase standard entropy (J·mol-1·K-1): 389.83
18. Gas phase standard formation free energy (kJ·mol-1): -39.0
19. Gas phase standard hot melt (J·mol-1 ·K-1):155.97
Toxicological data
1. Acute toxicity: LD50: 296 mg/kg (rat oral); LD50: 920 mg/kg (mouse transdermal); Transdermal); LD50: 450 mg/kg (orally in mice)
2. Steam is irritating to the eyes and respiratory mucosa
Ecological data
None
Molecular structure data
1. Molar refractive index: 37.78
2. Molar volume (cm3/mol): 120.4
3. Isotonic specific volume (90.2K): 297.5
4. Surface tension (dyne/cm): 37.2
5. Polarizability ( 10-24cm3): 14.97
6. Dipole moment (10-30C·m): 4.70
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Topological molecular polar surface area (TPSA): 20.2
6. Number of heavy atoms: 9
7. Surface charge: 0
8. Complexity: 80.6
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters : 0
11. Uncertain number of atomic stereocenters: 0
12. Determined number of chemical bond stereocenters: 0
13. Uncertain chemical bond formation Number of structural centers: 0
14. Number of covalent bond units: 1
Properties and stability
1. Corrosive and toxic. Can burn when exposed to open fire.
2. Exist in oriental tobacco leaves and smoke.
Storage method
None
Synthesis method
1. Use phenol or o-cresol as raw material, carry out gas phase catalytic reaction with methanol, and then purify through distillation. The purity of the product can reach more than 99%.
2. Obtain flake crystals or needle crystals from ethanol.
Purpose
Used in the production of polyphenylene ether resin, photographic chemicals, pesticides, polyester and polyether resin. The antiarrhythmic drug Slow Heart Rhythm can be prepared from 2,6-xylenol through hydroxypropylation, oxidative condensation, hydrogenation and salt formation.

 微信扫一扫打赏
微信扫一扫打赏

