
Structural formula
| Business number | 029G |
|---|---|
| Molecular formula | C8H11N |
| Molecular weight | 121 |
| label |
4-amino-o-xylene, 1-amino-3,4-dimethylbenzene, 4-Amino-o-xylene, 3,4-Dimethylaniline, 1-Amino-3,4-dimethylbenzene, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:95-64-7
MDL number:MFCD00007810
EINECS number:202-437-4
RTECS number:ZE9450000
BRN number:507414
PubChem number:24847724
Physical property data
1. Properties: The pure product is flake or columnar crystal, colorless to light reddish brown oily liquid.
2. Density (g/mL, 18℃): 1.076
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 49~51
5. Boiling point (ºC, normal pressure): 226
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 98
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V) : Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, chloroform, soluble in petroleum ether, ether, alcohol.
Toxicological data
1. Acute toxicity: rat oral LD50: 812mg/kg; mouse oral LD50: 707mg/kg; wild bird oral LD50: 5600μg/kg;
2. Mutagenicity Properties: Microbial Salmonella Typhimurium Mutations: 5 μmol/plate.
Ecological data
COD (Chemical Oxygen Demand): 30 This substance is harmful to the environment. Special attention should be paid to the pollution of water bodies. Since it is alkaline, special attention should be paid to plants. Special attention should also be paid to vegetables, soil and water organisms.
Molecular structure data
1. Molar refractive index: 40.13
2. Molar volume (cm3/mol): 124.2
3. Isotonic specific volume (90.2K ): 308.3
4. Surface tension (dyne/cm): 37.9
5. Polarizability (10-24cm3): 15.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 90.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong acids, acid anhydrides, acid chlorides, and halogens.
2. The oral LD50 of this product in mice is 707~812mg/kg. Oral ingestion and skin inhalation can cause poisoning.
3. Found in oriental tobacco leaves.
4. Highly toxic!
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from oxidants, acids, acid anhydrides, acid chlorides, and halogens, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills. Packed in 50kg iron drum. Store in a cool, ventilated warehouse, away from fire and heat sources, and away from direct sunlight. Store and transport separately from edible raw materials.
Synthesis method
1. 2-Chloromethyl-4-nitrotoluene is produced by chloromethylation of p-nitrotoluene with dichloromethyl ether, with a yield of 95%; then catalyze it with a nickel catalyst, and heat it at 35-30°C , hydrogenation at 3.5-4MPa produces 3,4-dimethylaniline. Another method is to use 3,4-dimethylacetophenone as raw material. It reacts with hydroxylamine hydrochloride and polyphosphoric acid successively, and finally hydrolyzes to obtain 3,4-dimethylaniline.
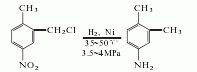
2. The preparation method is as follows It is produced by chloromethylation of p-nitrotoluene as raw material, followed by hydrogenation, reduction and dechlorination. Add dichloromethyl ether, p-nitrotoluene, and chlorosulfonic acid into the reaction kettle, stir and react at 15 to 20°C, then hydrolyze, filter, and wash the filter cake to obtain 2-chloromethyl-4-nitrotoluene. Dissolve 2-chloromethyl-4-nitrotoluene in ethanol and add nickel to the reactor. First, nitrogen gas is introduced to replace the air in the reactor, and then hydrogen gas is introduced. Control the temperature to 35-50°C and the pressure to 3.4-3.9 MPa. After the hydrogen is completed, ethanol is recovered by distillation, sodium hydroxide is added to alkalize, and the finished product is obtained by steam distillation.
3,4-dimethylaniline can also be prepared from o-xylene and starting materials.
Dissolve o-xylene in carbon disulfide, add anhydrous aluminum trichloride as a catalyst, and then add acetyl chloride dropwise. After the dropwise addition, react at 90°C for 30 minutes, then add hydrochloric acid, pour into ice water, and divide The aqueous solution was extracted with diethyl ether. The extract was washed with water, dried, evaporated to remove the diethyl ether, and distilled under reduced pressure to obtain 3,4-dimethylacetophenone. Then add 3,4-dimethylacetophenone to the mixture of hydroxylamine hydrochloride, water, potassium acetate, and methanol at a temperature of 40°C, then reflux in a water bath for 2 hours, pour into water, stir, cool, and precipitate Crystallize, filter, wash with water, and recrystallize with petroleum ether to obtain 2-(3,4-dimethylphenyl)acetoxime. Then heat the oximide and polyphosphoric acid in a water bath for 5 to 10 minutes to start to exotherm. Keep it at 120°C for 15 minutes. Recrystallize it from dilute ethanol to obtain acetyl 3,4-dimethylaniline. Then reflux with sulfuric acid and ethanol for 1.5 hours. Concentrate to half, add alkali to make it alkaline, extract with ether, dry, and evaporate the ether to obtain the finished product.
3. Tobacco: OR, 18.
Purpose
1. Used as dye intermediates and in organic synthesis.
2. Used as an intermediate for the pesticide pendimethalin and an intermediate for pharmaceutical vitamin B2.
extended-reading:https://www.newtopchem.com/archives/43979extended-reading:https://www.cyclohexylamine.net/benzyldimethylamine-nn-dimthylbenzylamine/extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/05/Lupragen-N205-MSDS.pdfextended-reading:https://www.newtopchem.com/archives/45187extended-reading:https://www.cyclohexylamine.net/cas-3033-62-3-bdmaee/extended-reading:https://www.bdmaee.net/dimethyltin-dioctanoate/extended-reading:https://www.newtopchem.com/archives/category/products/page/109extended-reading:https://www.bdmaee.net/nt-cat-bdma-catalyst-cas103-83-3-newtopchem/extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/3-9.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Methyl-Tin-Mercaptide-CAS26636-01-1-Coordinated-Thiol-Methyltin.pdf

 微信扫一扫打赏
微信扫一扫打赏

