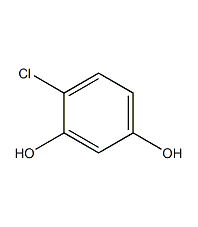
structural formula
| business number | 02a3 |
|---|---|
| molecular formula | c6h5clo2 |
| molecular weight | 144.56 |
| label |
4-chloro-1,3-benzenediol, 1,3-dihydroxy-4-chlorobenzene, 1-chloro-2,4-dihydroxybenzene, 4-chloro-1,3-dihydroxybenzene, 1,3-dihydroxy-4-chlorobenzene, 1-chloro-2,4-dihydroxybenzene, clc6h3(oh)2 |
numbering system
cas number:95-88-5
mdl number:mfcd00002273
einecs number:202-462-0
rtecs number:vh0450000
brn number:2042864
pubchem number:24892927
physical property data
1. properties: colorless crystals.
2. density (g/ml, 20℃): undetermined
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): 106-110
5. boiling point (ºc, normal pressure): 259
6. boiling point (ºc, 18mmhg): 147
7. refractive index: undetermined
8. flash point (ºc): undetermined
9. specific rotation (º): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (mmhg, ºc): undetermined
12. saturated vapor pressure (kpa , ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15 . critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) distribution coefficient: undetermined
17. explosion upper limit (%, v/v ): undetermined
18. lower explosion limit (%, v/v): undetermined
19. solubility: soluble in water, alcohol, ether, benzene and carbon disulfide.
toxicological data
1. skin/eye irritation
standard draize test: rabbit, eye contact: 5%, severity of reaction: mild.
2. acute toxicity: rat oral ld50: 369mg/kg; mouse abdominal ld50: 195mg/kg;
3. reproductive toxicity
rat oral tdlo: 2mg/kg (6-15 days after conception of female rats);
ecological data
none yet
molecular structure data
1. molar refractive index: 34.91
2. molar volume (cm3/mol): 98.2
3. isotonic specific volume (90.2k ): 273.1
4. surface tension (dyne/cm): 59.7
5. polarizability (10-24cm3): 13.83
compute chemical data
1.hydrophobic parameter metercalculation reference value (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 2
4. yes number of rotational chemical bonds: 0
5. number of tautomers: 9
6. topological molecule polar surface area 40.5
7. number of heavy atoms: 9
8. surface charge: 0
9. complexity: 97.1
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
none yet
synthesis method
derived from the reaction of resorcinol and dichlorosulfonyl. mix resorcinol and diethyl ether, stir and heat to reflux, and slowly add sulfur dichloride dropwise. then raise the temperature to 60°c and keep warm for 1 hour. after recovering the ether, distill it under normal pressure, then distill the distillate under reduced pressure, and collect the 131°c (0.8-0.93kpa) or 160-164°c (4.0kpa) fraction to obtain the finished product.
purpose
used for organic synthesis, preparation of various ether derivatives, drawings and analytical reagents.
extended-reading:https://www.cyclohexylamine.net/cell-improvement-agent-size-stabilizer/extended-reading:https://www.cyclohexylamine.net/category/product/page/27/extended-reading:https://www.bdmaee.net/pc-cat-nmm-catalyst/extended-reading:https://www.newtopchem.com/archives/44424extended-reading:https://www.morpholine.org/n-methylmorpholine/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33-12.jpgextended-reading:https://www.cyclohexylamine.net/cas-280-57-9-dabco-teda/extended-reading:https://www.bdmaee.net/cas23850-94-4/extended-reading:https://www.bdmaee.net/addocat-9558/extended-reading:https://www.bdmaee.net/niax-a-575-delayed-gel-type-tertiary-amine-catalyst-/

 微信扫一扫打赏
微信扫一扫打赏

