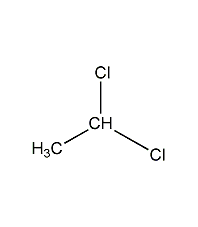
Structural formula
| Business number | 01JJ |
|---|---|
| Molecular formula | C2H4Cl2 |
| Molecular weight | 98.96 |
| label |
Ethylene dichloride, Ethylidene dichloride, 1,1-Ethylenedichloride, Ethylidene dichloride, Ethylidene chloride, as-Dichloroethane, CH3CHCl2, Low toxicity solvent, Heat-sensitive extractants |
Numbering system
CAS number:75-34-3
MDL number:MFCD00013673
EINECS number:200-863-5
RTECS number:KI0175000
BRN number:1696901
PubChem number:24863006
Physical property data
1. Properties: Colorless oily liquid with an ether smell and a saccharine sweetness. [1]
2. Melting point (℃): -97[2]
3. Boiling point (℃): 57.3[3]
4. Relative density (water = 1): 1.17[4]
5. Relative vapor Density (air=1): 3.92[5]
6. Saturated vapor pressure (kPa): 24.34 (20℃)[6]
7. Heat of combustion (kJ/mol): -1098.4[7]
8. Critical temperature (℃): 261.5[8]
9. Critical pressure (MPa): 5.05[9]
10. Octanol/water partition coefficient: 1.8 [10]
11. Flash point (℃): -17 (CC); 14 (OC) [11]
12 .Ignition temperature (℃): 458[12]
13. Explosion upper limit (%): 11.4[13]
14. Lower explosion limit (%): 5.6[14]
15. Solubility: Insoluble in water, soluble in most organic solvents. [15]
16. Viscosity (mPa·s, 20ºC): 0.4983
17. Flash point (ºC): 457.8
18. Heat of evaporation (KJ/mol, b.p.): 28.60
19. Heat of fusion (KJ/mol): 7.88
20. Heat of formation (KJ/mol, 20ºC, Liquid): 152.4
21. Heat of combustion (KJ/mol, 20ºC, liquid): 118.3
22. Specific heat capacity (KJ/(kg·K), 20ºC, liquid, constant Pressure): 1.28
23. Conductivity (S/m, 25ºC): <1.7×10-8
24. Relative density (25℃ , 4℃): 1.1679
25. Refractive index at room temperature (n25): 1.4138
26. Critical density (g·cm– 3): 0.42
27. Critical volume (cm3·mol-1): 236
28. Critical compression factor: 0.275
29. Eccentricity factor: 0.244
30. Lennard-Jones parameter (A): 8.628
31. Lennard-Jones Parameter (K): 241.2
32. Solubility parameter (J·cm-3)0.5: 18.330
33 .van der Waals area (cm2·mol-1): 6.330×109
34.van der Waals volume (cm3·mol-1): 44.930
35. Gas phase standard claims heat (enthalpy) (kJ·mol– 1): -130.1
36. Gas phase standard entropy (J·mol-1·K-1) ���305.17
37. Gas phase standard formation free energy (kJ·mol-1): -73.2
38. Gas phase standard hot melt (J· mol-1·K-1): 76.32
39. Liquid phase standard claims heat (enthalpy) (kJ·mol-1 ): -160.92
40. Liquid phase standard entropy (J·mol-1·K-1): 211.75 p>
41. Liquid phase standard free energy of formation (kJ·mol-1): -76.32
42. Liquid phase standard hot melt (J·mol-1·K-1): 126.27
Toxicological data
1. Acute toxicity[16]
LD50: 725mg/kg (rat oral)
LC50: 16000ppm (rat inhalation, 4h)
2. Irritation No data available
3. Asia Acute and chronic toxicity[17] Rats and guinea pigs inhaled 1000ppm, 6 hours a day, 5 days a week, 3 months, renal damage, increased urea nitrogen .
4. Mutagenicity[18] Sex chromosome deletion and non-disjunction: Aspergillus nidulans 2000ppm. Unprogrammed DNA synthesis: rat liver 13mmol/L
5. Teratogenicity[19] Rat pregnancy Inhalation of the lowest toxic dose (TCLo) of 6000ppm (7h) 6 to 15 days later can cause developmental malformations of the musculoskeletal system.
Ecological data
1. Ecotoxicity[20]
LC50: 550ppm (96h) (bluegill sunfish, Static); 480ppm (96h) (Moonfish, static)
2. Biodegradability[21]
Aerobic biodegradation (h): 768~3696
Anaerobic biodegradation (h): 3072~14784
3. Abiotic Degradability[22] Photooxidation half-life in air – high (h): 247~2468
4. Other harmful effects[23]
This substance may be harmful to the environment and bioaccumulates in food chains important to humans, especially in aquatic organisms.
Molecular structure data
1. Molar refractive index: 20.97
2. Molar volume (cm3/mol): 84.6
3. Isotonic specific volume (90.2K ): 185.9
4. Surface tension (dyne/cm): 23.2
5. Polarizability (10-24cm3): 8.31
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 11.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The solubility is similar to that of 1,2-dichloroethane, but the solubility of silicone resin in 1,1-dichloroethane is 20 times greater than that of 1,2-dichloroethane at 29°C. A flammable liquid that catches fire more easily than 1,2-dichloroethane and generates highly toxic phosgene when burned.
2. When this product undergoes chlorination reaction according to the free radical process in the liquid phase, 1,1,1-trichloroethane and 1,1,2-trichloroethane are roughly produced in a ratio of 3:1. Ethyl chloride. Dehydrochlorination produces vinyl chloride. In the presence of chlorine or water vapor, it is heated to above 300°C with metallic sodium to generate ethylene. It reacts with benzene in the presence of aluminum trichloride to produce 1,1-diphenylethane.
3. It is of low toxicity. Its toxicity to humans is similar to that of methyl chloride and chloroform, with strong local irritation and damage to the liver. In animal experiments, it was found that the cornea of the eyeball was cloudy. The maximum allowable concentration in the workplace is 400mg/m3 (Japan); 820mg/m3 (United States). The oral LD50 in rats is 14.1g/kg.
4. Stability[24] Stable
5. Incompatible substances[25] Strong oxidants, acids, alkalis
6. Conditions to avoid contact[26] Heating
7. Polymerization hazard[27] No polymerization
8. Decomposition products[28] Hydrogen chloride, phosgene
Storage method
Storage Precautions[29] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Industrially, it is obtained from the liquid phase reaction of vinyl chloride with hydrogen chloride under the catalysis of aluminum chloride, ferric chloride or zinc chloride, using 1,1-dichloroethane as the medium. When 1,2-dichloroethane is produced by chlorination of ethylene, a small amount of 1,1-dichloroethane is also produced as a by-product.
Purpose
1. Used as an extraction agent for solvents and heat-sensitive substances. Not as good as 1,2-� in industrial applicationsEthyl chloride is widely used. It is a low toxicity solvent. Used as raw material for manufacturing 1,1,1-trichloroethane.
2. Used as solvent, fumigant and intermediate in the production of 1,1,1-trichloroethane. [30]
extended-reading:https://www.bdmaee.net/dabco-tetn-catalyst-cas280-57-9-evonik-germany/
extended-reading:https://www.newtopchem.com/archives/947
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/bis3-dimethylaminopropyl-N-CAS-33329-35-0-Tris3-dimethylaminopropylamine.pdf
extended-reading:https://www.newtopchem.com/archives/43085
extended-reading:https://www.cyclohexylamine.net/dmcha-cas-98-94-2-n-dimethylcyclohexylamine/
extended-reading:https://www.newtopchem.com/archives/920
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33-14.jpg
extended-reading:https://www.bdmaee.net/pc-cat-np80-catalyst-trimethylhydroxyethyl-ethylene-diamine/
extended-reading:https://www.newtopchem.com/archives/category/products/page/31
extended-reading:https://www.bdmaee.net/low-odor-catalyst-9727/

 微信扫一扫打赏
微信扫一扫打赏

